Affinity chromatography offers high specificity by utilizing biomolecular interactions, making it ideal for purifying target proteins in biotechnology pet applications. Ion-exchange chromatography separates molecules based on charge differences, providing versatile purification options but with less specificity compared to affinity methods. Choosing between these techniques depends on the purity requirements and the nature of the biomolecules involved in the pet biotechnology process.
Table of Comparison
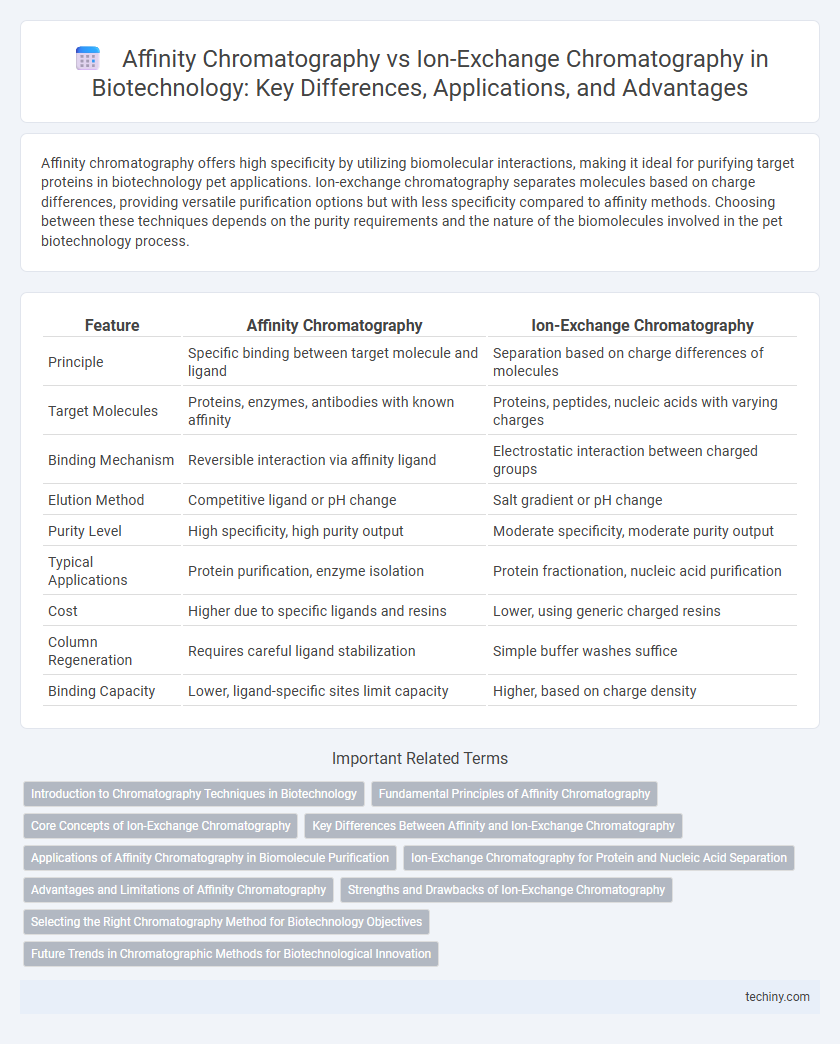
| Feature | Affinity Chromatography | Ion-Exchange Chromatography |
|---|---|---|
| Principle | Specific binding between target molecule and ligand | Separation based on charge differences of molecules |
| Target Molecules | Proteins, enzymes, antibodies with known affinity | Proteins, peptides, nucleic acids with varying charges |
| Binding Mechanism | Reversible interaction via affinity ligand | Electrostatic interaction between charged groups |
| Elution Method | Competitive ligand or pH change | Salt gradient or pH change |
| Purity Level | High specificity, high purity output | Moderate specificity, moderate purity output |
| Typical Applications | Protein purification, enzyme isolation | Protein fractionation, nucleic acid purification |
| Cost | Higher due to specific ligands and resins | Lower, using generic charged resins |
| Column Regeneration | Requires careful ligand stabilization | Simple buffer washes suffice |
| Binding Capacity | Lower, ligand-specific sites limit capacity | Higher, based on charge density |
Introduction to Chromatography Techniques in Biotechnology
Affinity chromatography exploits specific biochemical interactions between a target molecule and a ligand attached to the stationary phase, enabling highly selective purification of proteins or nucleic acids. Ion-exchange chromatography separates biomolecules based on their net surface charge by utilizing charged resins that interact with oppositely charged analytes, commonly applied for protein purification and desalting. These chromatography techniques are fundamental in biotechnology for isolating and analyzing biomolecules with high precision and yield.
Fundamental Principles of Affinity Chromatography
Affinity chromatography relies on specific biological interactions between a target molecule and a ligand immobilized on a stationary phase, enabling highly selective separation based on molecular recognition. This technique exploits reversible binding such as antigen-antibody, enzyme-substrate, or receptor-ligand interactions to isolate proteins, nucleic acids, or other biomolecules with high purity. Unlike ion-exchange chromatography, which separates molecules based on charge differences, affinity chromatography offers precise targeting of biomolecules through their unique functional groups or binding sites.
Core Concepts of Ion-Exchange Chromatography
Ion-exchange chromatography separates biomolecules based on their net surface charge by using charged resin beads that attract oppositely charged ions. The core concept involves exchanging ions in the mobile phase with those bound to the stationary phase, allowing selective binding and elution of proteins or nucleic acids. This technique is highly effective for purifying biomolecules by exploiting differences in their isoelectric points and charge density.
Key Differences Between Affinity and Ion-Exchange Chromatography
Affinity chromatography selectively isolates biomolecules based on specific binding interactions between the target molecule and ligands attached to the stationary phase, enabling high purity and specificity. Ion-exchange chromatography separates molecules according to their net surface charge by exploiting electrostatic attractions between charged analytes and oppositely charged resin groups, facilitating separation of proteins, peptides, and nucleotides with varying isoelectric points. The key differences involve affinity chromatography's use of highly specific biological interactions versus ion-exchange methods relying on charge properties for separation efficiency and selectivity in protein purification workflows.
Applications of Affinity Chromatography in Biomolecule Purification
Affinity chromatography excels in purifying biomolecules such as enzymes, antibodies, and recombinant proteins by exploiting specific binding interactions between the target molecule and ligands immobilized on the resin. This method achieves high selectivity and purity in applications like antibody isolation, protein complex purification, and enzyme purification from complex mixtures. Its ability to target specific biomolecular structures makes it indispensable for pharmaceutical manufacturing, diagnostics, and proteomics research.
Ion-Exchange Chromatography for Protein and Nucleic Acid Separation
Ion-exchange chromatography separates proteins and nucleic acids based on their net surface charge by exploiting interactions with charged resin matrices, making it highly effective for fractionating biomolecules with distinct isoelectric points. This technique allows precise adjustment of buffer pH and ionic strength to selectively elute bound molecules, enhancing purity and yield for downstream biotechnological applications. Ion-exchange chromatography is indispensable in protein purification workflows and nucleic acid isolation due to its scalability and high resolution.
Advantages and Limitations of Affinity Chromatography
Affinity chromatography offers high specificity and purity by exploiting biological interactions between the target molecule and a ligand, enabling efficient separation of proteins, enzymes, and antibodies. It allows for mild elution conditions, preserving the biological activity of sensitive biomolecules, while its limitations include high cost of affinity ligands and potential ligand leakage affecting product purity. Scale-up challenges and the need for prior knowledge of target-ligand interactions also restrict its applicability compared to ion-exchange chromatography.
Strengths and Drawbacks of Ion-Exchange Chromatography
Ion-exchange chromatography excels in separating proteins based on charge differences, offering high resolution and scalability for industrial bioprocessing. Its strength lies in the capacity to handle complex mixtures and achieve significant purification, but it often requires precise pH and ionic strength control to maintain protein stability and binding specificity. Limitations include potential protein denaturation and limited effectiveness with molecules that have similar charge properties or low charge density.
Selecting the Right Chromatography Method for Biotechnology Objectives
Affinity chromatography offers high specificity by targeting unique biomolecular interactions, ideal for purifying proteins with tagged ligands or antibodies. Ion-exchange chromatography separates molecules based on charge differences, providing versatility for protein mixtures with varied isoelectric points during purification. Selecting the right chromatography method depends on the target molecule's properties, desired purity levels, and scalability requirements in biotechnological applications.
Future Trends in Chromatographic Methods for Biotechnological Innovation
Affinity chromatography offers precise target molecule isolation by exploiting specific binding interactions, while ion-exchange chromatography provides versatile separation based on charge differences of biomolecules. Emerging trends emphasize integration of multi-modal chromatography combining affinity and ion-exchange principles to enhance selectivity, yield, and process scalability in biopharmaceutical manufacturing. Advances in smart materials and automation are driving continuous chromatographic processes, reducing costs and enabling real-time monitoring for next-generation biotechnological innovations.
Affinity chromatography vs Ion-exchange chromatography Infographic
 techiny.com
techiny.com