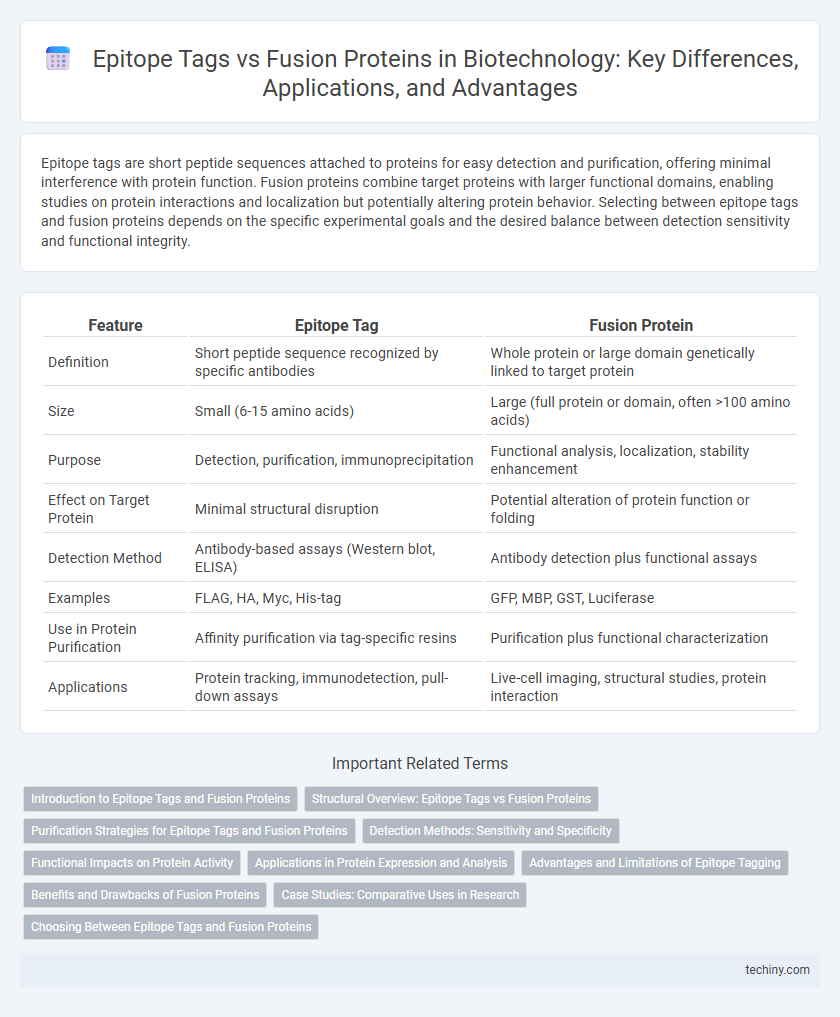
Epitope tags are short peptide sequences attached to proteins for easy detection and purification, offering minimal interference with protein function. Fusion proteins combine target proteins with larger functional domains, enabling studies on protein interactions and localization but potentially altering protein behavior. Selecting between epitope tags and fusion proteins depends on the specific experimental goals and the desired balance between detection sensitivity and functional integrity.
Table of Comparison
| Feature | Epitope Tag | Fusion Protein |
|---|---|---|
| Definition | Short peptide sequence recognized by specific antibodies | Whole protein or large domain genetically linked to target protein |
| Size | Small (6-15 amino acids) | Large (full protein or domain, often >100 amino acids) |
| Purpose | Detection, purification, immunoprecipitation | Functional analysis, localization, stability enhancement |
| Effect on Target Protein | Minimal structural disruption | Potential alteration of protein function or folding |
| Detection Method | Antibody-based assays (Western blot, ELISA) | Antibody detection plus functional assays |
| Examples | FLAG, HA, Myc, His-tag | GFP, MBP, GST, Luciferase |
| Use in Protein Purification | Affinity purification via tag-specific resins | Purification plus functional characterization |
| Applications | Protein tracking, immunodetection, pull-down assays | Live-cell imaging, structural studies, protein interaction |
Introduction to Epitope Tags and Fusion Proteins
Epitope tags are short peptide sequences genetically fused to proteins to enable detection, purification, and tracking using specific antibodies, enhancing experimental versatility. Fusion proteins combine functional domains from different proteins, allowing the study of protein interactions, localization, or therapeutic functions. Both tools are fundamental in biotechnology for protein analysis and manipulation, with epitope tags offering simpler detection and fusion proteins enabling multifunctional applications.
Structural Overview: Epitope Tags vs Fusion Proteins
Epitope tags are short peptide sequences engineered onto proteins to facilitate detection and purification, typically consisting of 6 to 12 amino acids, which minimally alter protein structure. Fusion proteins involve the covalent linking of a target protein with a larger protein or functional domain, often used to enhance solubility, stability, or enable multifunctional applications. Structural differences between epitope tags and fusion proteins critically influence protein folding, function, and downstream biotechnological applications such as affinity purification and antibody recognition.
Purification Strategies for Epitope Tags and Fusion Proteins
Epitope tags enable protein purification through affinity chromatography using tag-specific antibodies, allowing high specificity and mild elution conditions that preserve protein functionality. Fusion proteins integrate affinity tags such as His-tag or GST, facilitating purification via metal affinity or glutathione resin, with the added benefit of enhancing solubility and stability. Both strategies streamline the isolation process, but choice depends on desired purity, protein yield, and downstream applications.
Detection Methods: Sensitivity and Specificity
Epitope tags provide highly specific detection using antibodies that recognize short, unique peptide sequences, enabling sensitive immunodetection methods such as Western blotting and immunoprecipitation. Fusion proteins combine the target protein with a reporter or enzyme, offering versatile detection options including fluorescence, enzymatic activity assays, and immunodetection, often with enhanced sensitivity due to signal amplification. The choice between epitope tags and fusion proteins depends on the desired detection sensitivity, specificity, and experimental application in biotechnology workflows.
Functional Impacts on Protein Activity
Epitope tags, typically short peptide sequences like FLAG or HA, minimally interfere with protein folding or function due to their small size, making them ideal for detection without significantly altering protein activity. In contrast, fusion proteins, which involve larger domains such as GFP or GST, can significantly affect protein activity by altering localization, stability, or interaction dynamics. Understanding these functional impacts is crucial for experimental design in biotechnology to ensure accurate interpretation of protein behavior.
Applications in Protein Expression and Analysis
Epitope tags enable precise detection and purification of proteins by providing a specific antigenic sequence recognized by antibodies, facilitating rapid protein expression analysis in various systems. Fusion proteins combine the target protein with functional domains such as fluorescent proteins or affinity tags, enhancing solubility, stability, and enabling real-time visualization or purification. Both strategies are essential in recombinant protein research for optimizing expression, purification, and functional characterization in biotechnology workflows.
Advantages and Limitations of Epitope Tagging
Epitope tagging offers advantages such as facilitating the detection, purification, and localization of target proteins without disrupting their native function, thanks to small, well-characterized peptide sequences recognized by specific antibodies. Limitations include potential interference with protein folding or function if the tag is improperly positioned, and the possibility of immunogenic responses in certain applications. Compared to fusion proteins, epitope tags are less likely to alter protein behavior but may provide less functional versatility or stability enhancement.
Benefits and Drawbacks of Fusion Proteins
Fusion proteins offer enhanced functionality by combining multiple protein domains, enabling simultaneous purification, detection, and enzymatic activity. They can improve protein solubility and stability, facilitating structural and functional studies. However, fusion proteins may interfere with the native protein's function or folding, potentially altering biological activity and complicating experimental interpretations.
Case Studies: Comparative Uses in Research
Epitope tags are short peptide sequences used to detect or purify proteins with high specificity, frequently employed in immunoprecipitation and western blotting assays. Fusion proteins, created by genetically linking a protein of interest with a functional domain such as GFP or His-tag, enable both visualization and purification in live-cell imaging and affinity chromatography. Comparative case studies reveal epitope tags excel in minimal structural interference, while fusion proteins offer multifunctionality but may alter protein behavior, influencing experimental design in molecular biology research.
Choosing Between Epitope Tags and Fusion Proteins
Selecting between epitope tags and fusion proteins depends on the experimental goals and detection requirements in biotechnology. Epitope tags, such as FLAG or HA tags, offer minimal interference with protein function and enable specific antibody recognition for purification or imaging. Fusion proteins, combining a target protein with reporters like GFP or enzymes, provide functional or localization insights but may affect protein folding or activity, necessitating careful evaluation of tag placement and size.
Epitope tag vs Fusion protein Infographic
 techiny.com
techiny.com