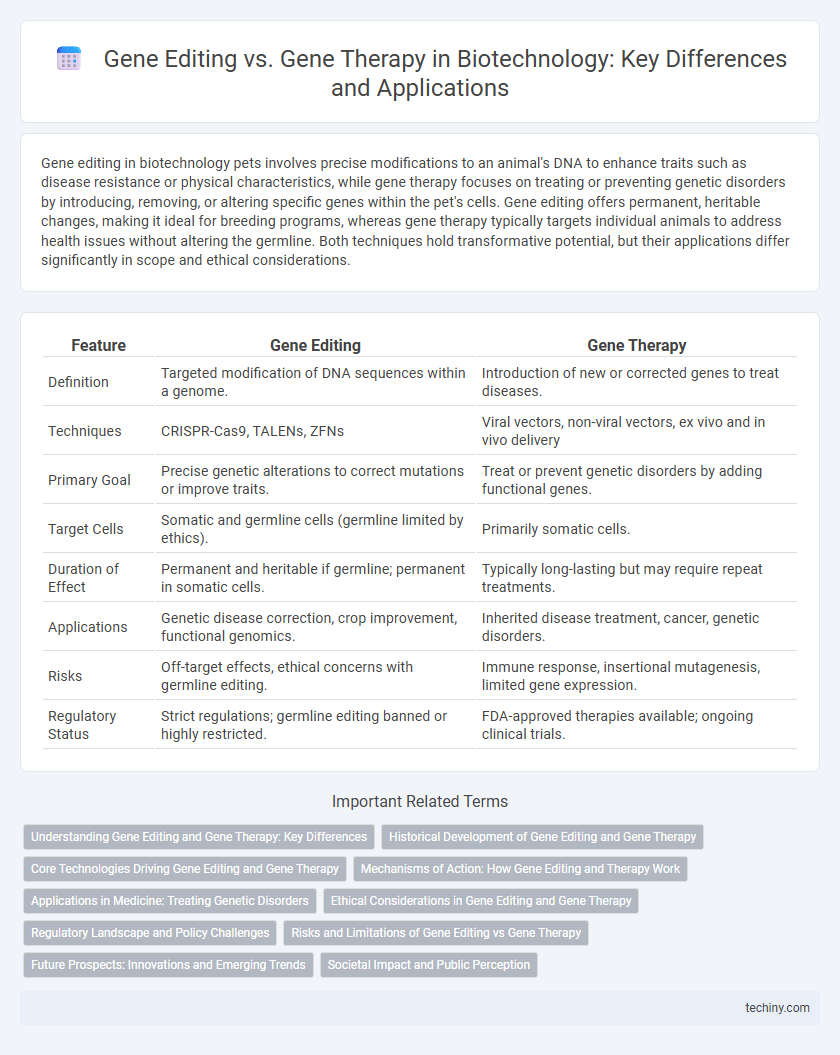
Gene editing in biotechnology pets involves precise modifications to an animal's DNA to enhance traits such as disease resistance or physical characteristics, while gene therapy focuses on treating or preventing genetic disorders by introducing, removing, or altering specific genes within the pet's cells. Gene editing offers permanent, heritable changes, making it ideal for breeding programs, whereas gene therapy typically targets individual animals to address health issues without altering the germline. Both techniques hold transformative potential, but their applications differ significantly in scope and ethical considerations.
Table of Comparison
| Feature | Gene Editing | Gene Therapy |
|---|---|---|
| Definition | Targeted modification of DNA sequences within a genome. | Introduction of new or corrected genes to treat diseases. |
| Techniques | CRISPR-Cas9, TALENs, ZFNs | Viral vectors, non-viral vectors, ex vivo and in vivo delivery |
| Primary Goal | Precise genetic alterations to correct mutations or improve traits. | Treat or prevent genetic disorders by adding functional genes. |
| Target Cells | Somatic and germline cells (germline limited by ethics). | Primarily somatic cells. |
| Duration of Effect | Permanent and heritable if germline; permanent in somatic cells. | Typically long-lasting but may require repeat treatments. |
| Applications | Genetic disease correction, crop improvement, functional genomics. | Inherited disease treatment, cancer, genetic disorders. |
| Risks | Off-target effects, ethical concerns with germline editing. | Immune response, insertional mutagenesis, limited gene expression. |
| Regulatory Status | Strict regulations; germline editing banned or highly restricted. | FDA-approved therapies available; ongoing clinical trials. |
Understanding Gene Editing and Gene Therapy: Key Differences
Gene editing involves precise modification of an organism's DNA sequences using tools like CRISPR-Cas9 to directly alter genetic material at specific locations. Gene therapy, on the other hand, introduces, removes, or alters genetic material within a patient's cells to treat or prevent disease, often by delivering functional genes via vectors such as viral carriers. While gene editing enables targeted genetic corrections, gene therapy focuses on the functional replacement or supplementation of defective genes to achieve therapeutic outcomes.
Historical Development of Gene Editing and Gene Therapy
Gene editing techniques originated with the discovery of restriction enzymes in the 1970s and advanced significantly with the development of CRISPR-Cas9 technology in 2012, enabling precise genomic modifications. Gene therapy's historical development began in the 1990s with successful delivery of functional genes using viral vectors to treat inherited disorders, marking a shift towards clinical applications. Both fields have evolved through breakthroughs in molecular biology and genome engineering, shaping modern strategies for treating genetic diseases.
Core Technologies Driving Gene Editing and Gene Therapy
Core technologies driving gene editing include CRISPR-Cas9, TALENs, and zinc-finger nucleases, which enable precise DNA modifications at target genomic sites. In gene therapy, viral vectors such as adeno-associated viruses (AAV) and lentiviruses are essential for delivering therapeutic genes into patient cells for long-term expression. Advances in delivery systems and genome editing tools are accelerating the development of personalized medicine and treatment of genetic disorders.
Mechanisms of Action: How Gene Editing and Therapy Work
Gene editing utilizes precise molecular tools such as CRISPR-Cas9 to directly alter the DNA sequence at specific genomic locations, enabling targeted modifications like gene knock-in, knockout, or correction. Gene therapy typically involves the delivery of functional genes into patient cells via viral or non-viral vectors to compensate for defective or missing genes, often without altering the genome permanently. While gene editing modifies the genetic code at the DNA level, gene therapy primarily introduces therapeutic genes as functional payloads to restore or enhance cellular function.
Applications in Medicine: Treating Genetic Disorders
Gene editing technologies, such as CRISPR-Cas9, enable precise modifications of DNA sequences to correct genetic mutations at their source, offering curative potential for inherited disorders like cystic fibrosis and sickle cell anemia. Gene therapy, typically involving the delivery of functional genes via viral vectors, addresses diseases by supplementing or replacing defective genes, proving effective in conditions like hemophilia and certain immunodeficiencies. Both approaches revolutionize medical treatment paradigms by targeting the molecular basis of genetic disorders, significantly improving patient outcomes and quality of life.
Ethical Considerations in Gene Editing and Gene Therapy
Gene editing raises ethical concerns related to unintended genetic consequences and germline modifications that can affect future generations, necessitating strict regulatory oversight to prevent misuse and ensure patient safety. Gene therapy, while primarily targeting somatic cells, poses ethical questions over informed consent, equitable access, and long-term effects on treated individuals. Both fields demand transparent ethical frameworks to balance innovation with societal impact, preventing discrimination and safeguarding human rights.
Regulatory Landscape and Policy Challenges
Gene editing technologies such as CRISPR face distinct regulatory scrutiny compared to traditional gene therapy, with agencies emphasizing off-target effects and germline modifications. Regulatory frameworks vary globally, with countries like the US adopting case-by-case evaluations, while the EU enforces stricter precautionary principles. Policy challenges include ethical concerns, accessibility disparities, and the need for international harmonization to ensure safe and equitable applications in biotechnology.
Risks and Limitations of Gene Editing vs Gene Therapy
Gene editing techniques, such as CRISPR-Cas9, present risks including off-target mutations and unintended genetic consequences that may lead to tumorigenesis or immune responses. In contrast, gene therapy often faces limitations in delivery efficiency, potential insertional mutagenesis, and transient therapeutic effects due to immune clearance of viral vectors. Both approaches require careful evaluation of long-term safety and ethical considerations to mitigate unforeseen adverse outcomes.
Future Prospects: Innovations and Emerging Trends
Gene editing technologies like CRISPR-Cas9 offer unprecedented precision in manipulating genetic sequences, enabling advancements in customized treatments for genetic disorders and complex diseases. Emerging trends include base editing and prime editing, which promise reduced off-target effects and enhanced safety profiles compared to traditional gene therapy approaches. Innovations in delivery systems, such as lipid nanoparticles and viral vectors, are expanding the therapeutic potential and clinical applications of both gene editing and gene therapy in personalized medicine.
Societal Impact and Public Perception
Gene editing technologies like CRISPR enable precise alterations to DNA, raising concerns about ethical boundaries and potential long-term consequences on future generations. Public perception varies, with gene therapy often viewed more favorably due to its focus on treating existing genetic disorders rather than altering the hereditary genome. Societal impact centers on balancing medical innovation with regulatory frameworks to ensure equitable access and prevent misuse.
gene editing vs gene therapy Infographic
 techiny.com
techiny.com