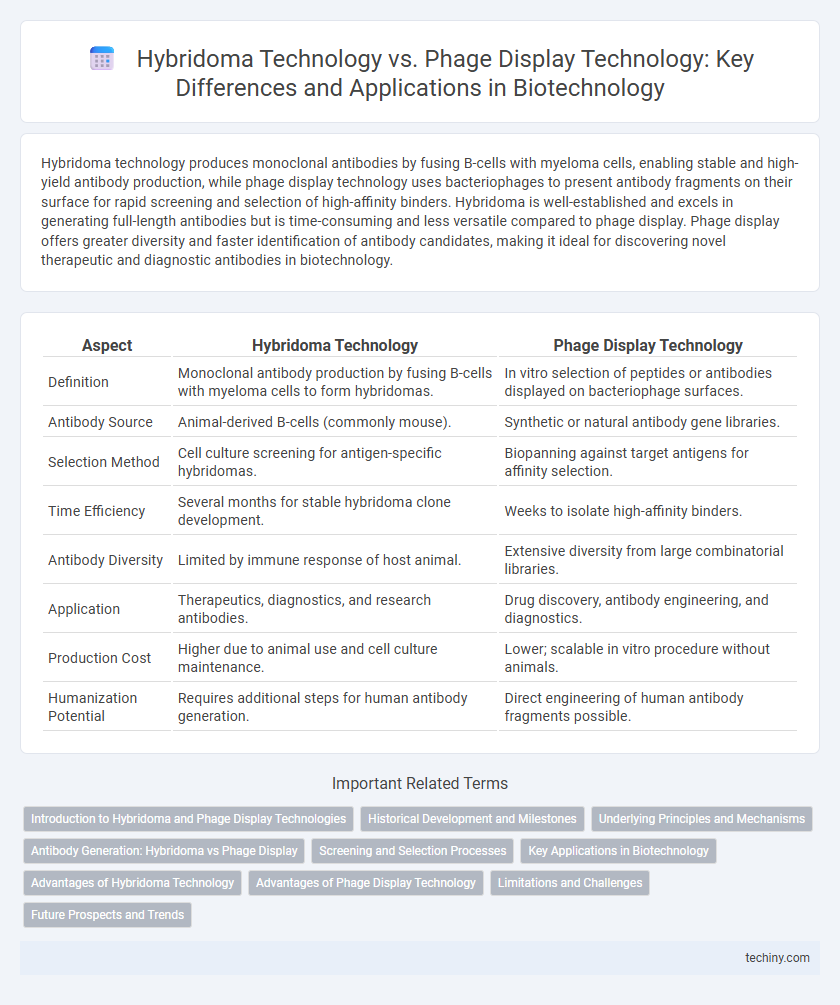
Hybridoma technology produces monoclonal antibodies by fusing B-cells with myeloma cells, enabling stable and high-yield antibody production, while phage display technology uses bacteriophages to present antibody fragments on their surface for rapid screening and selection of high-affinity binders. Hybridoma is well-established and excels in generating full-length antibodies but is time-consuming and less versatile compared to phage display. Phage display offers greater diversity and faster identification of antibody candidates, making it ideal for discovering novel therapeutic and diagnostic antibodies in biotechnology.
Table of Comparison
| Aspect | Hybridoma Technology | Phage Display Technology |
|---|---|---|
| Definition | Monoclonal antibody production by fusing B-cells with myeloma cells to form hybridomas. | In vitro selection of peptides or antibodies displayed on bacteriophage surfaces. |
| Antibody Source | Animal-derived B-cells (commonly mouse). | Synthetic or natural antibody gene libraries. |
| Selection Method | Cell culture screening for antigen-specific hybridomas. | Biopanning against target antigens for affinity selection. |
| Time Efficiency | Several months for stable hybridoma clone development. | Weeks to isolate high-affinity binders. |
| Antibody Diversity | Limited by immune response of host animal. | Extensive diversity from large combinatorial libraries. |
| Application | Therapeutics, diagnostics, and research antibodies. | Drug discovery, antibody engineering, and diagnostics. |
| Production Cost | Higher due to animal use and cell culture maintenance. | Lower; scalable in vitro procedure without animals. |
| Humanization Potential | Requires additional steps for human antibody generation. | Direct engineering of human antibody fragments possible. |
Introduction to Hybridoma and Phage Display Technologies
Hybridoma technology involves the fusion of antibody-producing B cells with immortal myeloma cells to create hybrid cells that continuously produce monoclonal antibodies, enabling precise targeting of specific antigens. Phage display technology uses bacteriophages to express antibody fragments on their surface, allowing rapid screening and selection of high-affinity binders from vast peptide or antibody libraries. Both methods revolutionize antibody development by enabling the production of highly specific and scalable monoclonal antibodies for diagnostics and therapeutics.
Historical Development and Milestones
Hybridoma technology, pioneered in 1975 by Kohler and Milstein, revolutionized monoclonal antibody production by fusing myeloma cells with antibody-producing B cells, enabling the creation of immortalized antibody-secreting cell lines. In contrast, phage display technology, developed in the mid-1980s by George P. Smith, introduced a genetic approach where bacteriophages display peptides or proteins on their surface, facilitating rapid identification of high-affinity antibodies through in vitro selection. Both technologies have significantly advanced therapeutic antibody discovery, with hybridomas dominating early commercial antibody production and phage display providing enhanced diversity and affinity maturation capabilities in modern biotechnology.
Underlying Principles and Mechanisms
Hybridoma technology relies on fusing antibody-producing B cells with myeloma cells to create hybrid cells capable of continuous monoclonal antibody production, utilizing the natural immune response mechanism. Phage display technology employs bacteriophages to present antibody fragments on their surface, enabling the selection of high-affinity binders through iterative rounds of binding and amplification, based on in vitro combinatorial library screening. Both methods aim to generate specific antibodies but differ fundamentally in their cellular versus molecular display and selection processes.
Antibody Generation: Hybridoma vs Phage Display
Hybridoma technology produces monoclonal antibodies by fusing B-cells with myeloma cells, enabling stable and continuous antibody secretion, while phage display technology generates antibody libraries displayed on bacteriophages, facilitating rapid screening of high-affinity antibody fragments. Hybridoma-derived antibodies often have high specificity and stability but require animal immunization and longer development times. Phage display offers greater diversity and faster selection cycles, allowing in vitro evolution and humanization of antibodies without the need for animal hosts.
Screening and Selection Processes
Hybridoma technology utilizes the fusion of antibody-producing B cells with myeloma cells, enabling the screening of monoclonal antibodies through ELISA or flow cytometry to identify high-affinity clones. Phage display technology involves the presentation of antibody fragments on bacteriophages, allowing for the iterative panning and selection against specific antigens to isolate peptides with desired binding properties. The selection process in phage display is typically faster and allows for the exploration of larger combinatorial libraries compared to the hybridoma method.
Key Applications in Biotechnology
Hybridoma technology is primarily used for producing monoclonal antibodies for diagnostic and therapeutic applications, including cancer treatment and autoimmune disease management. Phage display technology excels in identifying peptides, proteins, and antibody fragments with high affinity, crucial for drug discovery, vaccine development, and protein engineering. Both technologies are essential in biotechnology, with hybridoma offering stable antibody production and phage display enabling rapid screening of vast molecular libraries.
Advantages of Hybridoma Technology
Hybridoma technology offers the advantage of producing highly specific monoclonal antibodies with consistent affinity and specificity due to the stable fusion of B-cells with myeloma cells. This method enables large-scale antibody production with reliable reproducibility, essential for diagnostic and therapeutic applications. Unlike phage display, hybridoma technology maintains natural antibody folding and post-translational modifications, enhancing antibody functionality and efficacy.
Advantages of Phage Display Technology
Phage display technology offers a significant advantage over hybridoma technology by enabling the rapid screening of vast peptide or antibody libraries, often exceeding 10^9 variants, facilitating the discovery of high-affinity binders. Unlike hybridoma methods that rely on animal immunization and fusion processes, phage display is entirely in vitro, allowing greater control over selection conditions and the ability to engineer antibodies with enhanced specificity and affinity. This technology also provides a cost-effective and scalable platform for antibody development, expediting therapeutic and diagnostic applications in biotechnology.
Limitations and Challenges
Hybridoma technology faces limitations such as low antibody diversity, time-consuming procedures, and difficulty in producing humanized antibodies, which can lead to immunogenicity issues in therapeutic applications. Phage display technology encounters challenges including the risk of biased library representation, instability of displayed peptides or proteins, and the requirement for extensive screening to isolate high-affinity binders. Both methods demand careful optimization to overcome bottlenecks related to efficiency, specificity, and scalability in antibody generation.
Future Prospects and Trends
Hybridoma technology continues to advance with innovations in monoclonal antibody production, emphasizing enhanced specificity and reduced immunogenicity for therapeutic applications. Phage display technology is rapidly evolving through integration with next-generation sequencing and machine learning, enabling faster identification of high-affinity antibodies and peptides. Future trends point towards combining both platforms for personalized medicine, improving antibody engineering efficiency, and expanding applications in diagnostics and targeted drug delivery.
**Hybridoma technology vs Phage display technology** Infographic
 techiny.com
techiny.com