Microfluidics offers precise control over fluid manipulation at the microscale, enabling high-throughput screening and reduced reagent consumption compared to conventional bioprocessing. This technology enhances cell culture environments by providing uniform nutrient delivery and waste removal, improving reproducibility and scalability in biotechnology applications. Conventional bioprocessing remains valuable for large-volume production but often faces challenges with heterogeneity and resource inefficiency.
Table of Comparison
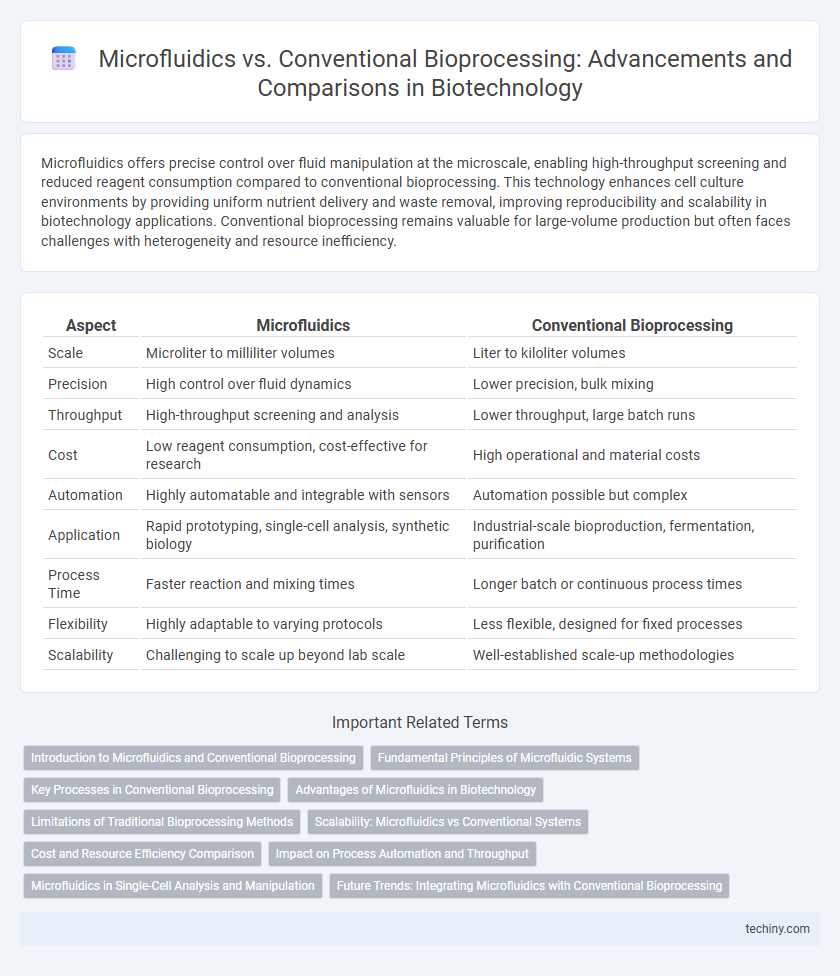
| Aspect | Microfluidics | Conventional Bioprocessing |
|---|---|---|
| Scale | Microliter to milliliter volumes | Liter to kiloliter volumes |
| Precision | High control over fluid dynamics | Lower precision, bulk mixing |
| Throughput | High-throughput screening and analysis | Lower throughput, large batch runs |
| Cost | Low reagent consumption, cost-effective for research | High operational and material costs |
| Automation | Highly automatable and integrable with sensors | Automation possible but complex |
| Application | Rapid prototyping, single-cell analysis, synthetic biology | Industrial-scale bioproduction, fermentation, purification |
| Process Time | Faster reaction and mixing times | Longer batch or continuous process times |
| Flexibility | Highly adaptable to varying protocols | Less flexible, designed for fixed processes |
| Scalability | Challenging to scale up beyond lab scale | Well-established scale-up methodologies |
Introduction to Microfluidics and Conventional Bioprocessing
Microfluidics technology manipulates fluids at the microliter to picoliter scale, enabling precise control and high-throughput analysis in bioprocessing. Conventional bioprocessing typically involves larger-scale reactors and batch processing, which can limit efficiency and scalability. Microfluidics offers enhanced reaction kinetics, reduced reagent consumption, and improved process integration compared to traditional methods.
Fundamental Principles of Microfluidic Systems
Microfluidic systems operate by precisely manipulating fluids at the microscale using laminar flow, surface tension, and diffusion-driven transport, enabling high control over reaction conditions. Unlike conventional bioprocessing, which relies on bulk mixing and large volumes, microfluidics facilitates rapid heat and mass transfer through microchannel networks and low Reynolds number flows. These fundamental principles allow for enhanced cell culture, biomolecule analysis, and drug discovery with improved efficiency and scalability.
Key Processes in Conventional Bioprocessing
Conventional bioprocessing relies on large-scale batch or fed-batch fermentation systems for microbial or cell culture growth, emphasizing strict control over parameters like pH, temperature, and dissolved oxygen to optimize product yield. Key processes include upstream biomass cultivation, downstream cell harvesting, and product purification through filtration, centrifugation, and chromatography techniques. These steps are critical for maintaining process robustness and scalability in producing biologics such as monoclonal antibodies, vaccines, and enzymes.
Advantages of Microfluidics in Biotechnology
Microfluidics enables precise control over cellular environments, enhancing reaction efficiency and reducing reagent consumption significantly compared to conventional bioprocessing. The technology facilitates high-throughput screening and single-cell analysis, accelerating biomolecule discovery and personalized medicine development. Reduced sample volumes and integration with automation systems improve scalability and reproducibility in biotechnological applications.
Limitations of Traditional Bioprocessing Methods
Traditional bioprocessing methods face limitations such as large reagent volumes, slower reaction times, and reduced control over microenvironmental conditions during cell culture or biochemical reactions. These constraints hinder scalability, increase cost, and limit experimental reproducibility compared to microfluidics, which offers precise fluid handling at the microscale. Inefficiencies in mass transfer and mixing further challenge conventional bioprocesses in achieving optimal yields and consistent product quality.
Scalability: Microfluidics vs Conventional Systems
Microfluidics offers precise control at microscale levels but faces challenges in scaling up for industrial bioprocessing due to limited throughput. Conventional bioprocessing systems, such as stirred-tank reactors, provide established scalability with volumes from liters to thousands of cubic meters, enabling mass production. Integrating microfluidic technology into conventional platforms aims to enhance efficiency and control while maintaining scalable output.
Cost and Resource Efficiency Comparison
Microfluidics technology significantly reduces reagent consumption and waste generation compared to conventional bioprocessing, leading to lower operational costs and improved resource efficiency. The miniaturization and automation inherent in microfluidic systems enable high-throughput screening with minimal sample volumes, significantly decreasing material expenses and labor requirements. Conventional bioprocessing often involves larger-scale equipment and bulk reagents, resulting in higher energy consumption and increased resource use, making microfluidics a cost-effective alternative for scalable biotechnological applications.
Impact on Process Automation and Throughput
Microfluidics technology significantly enhances process automation by enabling precise control of fluid dynamics on a microscale, facilitating high-throughput screening and real-time monitoring with minimal reagent consumption. Conventional bioprocessing often faces limitations in scalability and automation due to larger volumes and complex mechanical setups, leading to slower throughput and increased risk of contamination. Integration of microfluidics into bioprocessing workflows accelerates experimental iterations and data acquisition, improving efficiency and consistency in biotechnological production and research.
Microfluidics in Single-Cell Analysis and Manipulation
Microfluidics offers unparalleled precision in single-cell analysis and manipulation by enabling the isolation, sorting, and real-time monitoring of individual cells within micro-scale channels. This technology enhances throughput and sensitivity compared to conventional bioprocessing techniques, allowing for detailed cellular profiling, drug screening, and genetic analysis at a single-cell level. Integration of microfluidic platforms with advanced imaging and molecular diagnostics drives breakthroughs in personalized medicine and targeted therapies.
Future Trends: Integrating Microfluidics with Conventional Bioprocessing
Integrating microfluidics with conventional bioprocessing accelerates high-throughput screening and enhances precise control over cell cultures and reactions, driving scalability in biomanufacturing. Advances in microfluidic chip design enable seamless coupling with large-scale bioreactors, reducing reagent consumption and enabling real-time monitoring of biological variables. Future trends emphasize hybrid platforms combining microfluidic precision with robust conventional bioprocessing for improved yield, cost-efficiency, and process automation in biotechnology.
Microfluidics vs Conventional Bioprocessing Infographic
 techiny.com
techiny.com