Phage display and yeast two-hybrid are powerful biotechnology techniques used to study protein interactions, each with unique advantages. Phage display involves expressing protein libraries on bacteriophage surfaces to identify binding partners efficiently, making it ideal for selecting high-affinity peptides and antibodies. Yeast two-hybrid employs a genetic system within yeast cells to detect protein-protein interactions in vivo, providing detailed insights into cellular interaction networks and functional relationships.
Table of Comparison
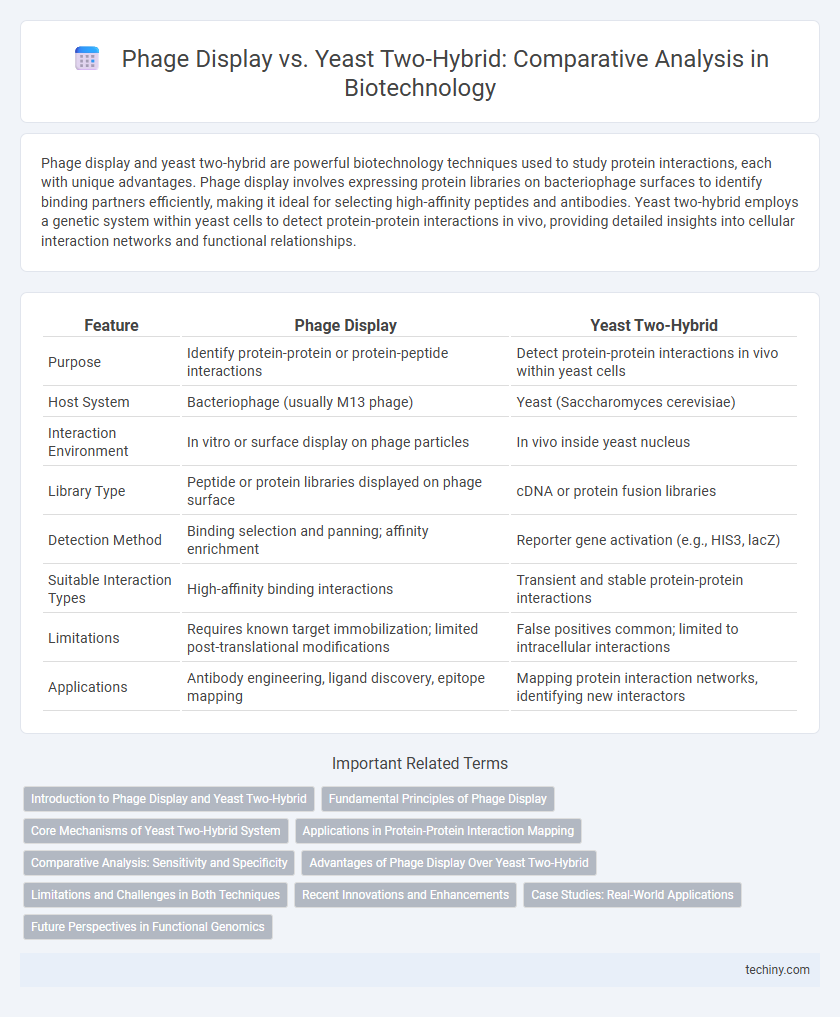
| Feature | Phage Display | Yeast Two-Hybrid |
|---|---|---|
| Purpose | Identify protein-protein or protein-peptide interactions | Detect protein-protein interactions in vivo within yeast cells |
| Host System | Bacteriophage (usually M13 phage) | Yeast (Saccharomyces cerevisiae) |
| Interaction Environment | In vitro or surface display on phage particles | In vivo inside yeast nucleus |
| Library Type | Peptide or protein libraries displayed on phage surface | cDNA or protein fusion libraries |
| Detection Method | Binding selection and panning; affinity enrichment | Reporter gene activation (e.g., HIS3, lacZ) |
| Suitable Interaction Types | High-affinity binding interactions | Transient and stable protein-protein interactions |
| Limitations | Requires known target immobilization; limited post-translational modifications | False positives common; limited to intracellular interactions |
| Applications | Antibody engineering, ligand discovery, epitope mapping | Mapping protein interaction networks, identifying new interactors |
Introduction to Phage Display and Yeast Two-Hybrid
Phage display is a powerful molecular technique that involves expressing peptides or proteins on the surface of bacteriophages to identify protein-protein interactions and screen large libraries for binding affinity. Yeast two-hybrid is a genetic method used to detect protein-protein interactions within a eukaryotic cellular environment by reconstituting a functional transcription factor when two proteins of interest bind. Both methods are widely used in biotechnology for mapping interaction networks, but phage display offers advantages in peptide selection, while yeast two-hybrid excels in studying intracellular protein interactions.
Fundamental Principles of Phage Display
Phage display is a powerful molecular biology technique that involves expressing peptides or proteins on the surface of bacteriophages to study protein-protein, protein-peptide, and protein-DNA interactions. This method relies on linking the genotype (phage DNA) to the phenotype (displayed protein), enabling rapid screening and selection of binders from vast combinatorial libraries. The fundamental principle of phage display centers on the fusion of a gene encoding a protein of interest to a phage coat protein gene, resulting in the displayed protein being physically attached to its encoding DNA within the viral particle.
Core Mechanisms of Yeast Two-Hybrid System
The yeast two-hybrid system operates by detecting protein-protein interactions through the reconstitution of a functional transcription factor when two hybrid proteins bind, enabling the activation of reporter genes. This method involves fusing a bait protein to a DNA-binding domain and a prey protein to a transcriptional activation domain, allowing interaction-driven transcriptional activation within yeast nuclei. Its core mechanism emphasizes in vivo identification of binary protein interactions, which contrasts with the phage display technique's in vitro selection of peptide or protein ligands on phage surfaces.
Applications in Protein-Protein Interaction Mapping
Phage display and yeast two-hybrid systems are powerful tools for mapping protein-protein interactions, each with distinct advantages in biotechnology. Phage display enables the identification of peptide ligands and antibody fragments with high affinity through large combinatorial libraries, making it ideal for therapeutic target discovery and epitope mapping. Yeast two-hybrid technology facilitates in vivo detection of binary protein interactions within a eukaryotic cellular environment, supporting the elucidation of interaction networks and functional proteomics.
Comparative Analysis: Sensitivity and Specificity
Phage display exhibits higher sensitivity by enabling the screening of vast peptide or protein libraries through binding affinity selection, while yeast two-hybrid systems detect protein-protein interactions within a cellular context, enhancing biological relevancy but often with lower sensitivity. Specificity in phage display is driven by stringent binding conditions and repeated selection rounds, reducing false positives, whereas yeast two-hybrid assays may suffer from higher false-positive rates due to non-specific interactions in the nuclear environment. Both methods offer complementary strengths: phage display excels in isolating high-affinity ligands with precise target recognition, whereas yeast two-hybrid prioritizes functional interaction mapping at the cost of sensitivity and specificity balance.
Advantages of Phage Display Over Yeast Two-Hybrid
Phage display offers higher library diversity and selection efficiency compared to yeast two-hybrid, enabling rapid identification of high-affinity peptides and antibodies. It allows direct binding interactions with immobilized targets, bypassing the need for intracellular expression, which can limit yeast two-hybrid performance. Additionally, phage display facilitates the screening of a wider range of protein-protein interactions, including those involving membrane proteins and toxic peptides.
Limitations and Challenges in Both Techniques
Phage display is limited by the inability to represent complex post-translational modifications and membrane proteins, often resulting in false positives due to non-specific binding. Yeast two-hybrid systems face challenges with detecting interactions involving membrane-bound proteins or proteins requiring native mammalian cellular contexts, and it frequently yields false negatives because of improper folding or localization in yeast cells. Both techniques struggle with high-throughput screening accuracy and the interpretation of weak or transient protein-protein interactions.
Recent Innovations and Enhancements
Recent innovations in Phage display include engineered phage libraries with enhanced diversity and binding affinity, enabling more precise antibody discovery and protein interaction studies. Yeast two-hybrid systems have advanced through high-throughput screening automation and multiplexed interaction mapping, improving the identification of complex protein-protein networks. Both technologies benefit from integration with next-generation sequencing and machine learning algorithms to increase sensitivity and reduce false positives in interaction detection.
Case Studies: Real-World Applications
Phage display technology has been effectively utilized in the development of therapeutic antibodies, such as in the case of Adalimumab for autoimmune diseases, showcasing its strength in ligand identification and affinity maturation. Yeast two-hybrid systems have been instrumental in mapping protein-protein interactions within complex cellular pathways, exemplified by their use in studying cancer-related signaling networks like the p53 tumor suppressor pathway. Both techniques provide complementary insights in drug discovery and functional genomics, with phage display excelling in extracellular target binding and yeast two-hybrid excelling in intracellular interaction analysis.
Future Perspectives in Functional Genomics
Phage display offers high-throughput screening of protein interactions and peptide libraries, enabling rapid identification of binding partners crucial for drug development and antibody engineering. Yeast two-hybrid systems provide in vivo detection of protein-protein interactions within a cellular context, facilitating the mapping of complex interaction networks and functional pathways. Future advancements integrating phage display's peptide diversity with yeast two-hybrid's intracellular environment promise enhanced precision in functional genomics and therapeutic target discovery.
**Phage display vs Yeast two-hybrid** Infographic
 techiny.com
techiny.com