Prokaryotic expression systems offer rapid protein production and cost-efficiency, making them ideal for expressing simple proteins without complex post-translational modifications. Eukaryotic expression systems provide the necessary machinery for proper folding, glycosylation, and functional expression of complex proteins often required in biopharmaceuticals. Selecting between prokaryotic and eukaryotic systems depends on the protein's structural requirements and intended application in biotechnology.
Table of Comparison
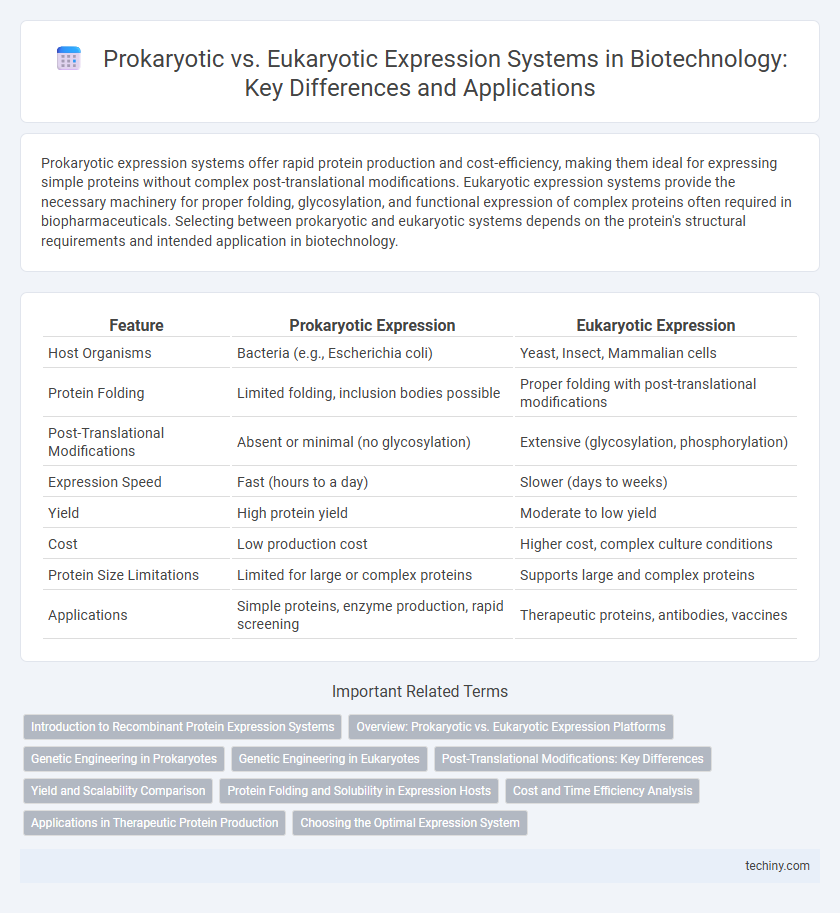
| Feature | Prokaryotic Expression | Eukaryotic Expression |
|---|---|---|
| Host Organisms | Bacteria (e.g., Escherichia coli) | Yeast, Insect, Mammalian cells |
| Protein Folding | Limited folding, inclusion bodies possible | Proper folding with post-translational modifications |
| Post-Translational Modifications | Absent or minimal (no glycosylation) | Extensive (glycosylation, phosphorylation) |
| Expression Speed | Fast (hours to a day) | Slower (days to weeks) |
| Yield | High protein yield | Moderate to low yield |
| Cost | Low production cost | Higher cost, complex culture conditions |
| Protein Size Limitations | Limited for large or complex proteins | Supports large and complex proteins |
| Applications | Simple proteins, enzyme production, rapid screening | Therapeutic proteins, antibodies, vaccines |
Introduction to Recombinant Protein Expression Systems
Prokaryotic expression systems, primarily using Escherichia coli, offer rapid growth and high-yield recombinant protein production but may lack essential post-translational modifications. Eukaryotic expression systems, such as yeast, insect, and mammalian cells, enable proper protein folding and complex modifications crucial for functional recombinant proteins. Selecting the appropriate system depends on protein complexity, desired yield, and functional activity requirements.
Overview: Prokaryotic vs. Eukaryotic Expression Platforms
Prokaryotic expression platforms, primarily utilizing Escherichia coli, offer rapid protein production, cost-effectiveness, and straightforward genetic manipulation but often lack post-translational modifications essential for functional eukaryotic proteins. Eukaryotic expression systems, including yeast, insect, and mammalian cells, enable complex folding and post-translational modifications such as glycosylation, crucial for therapeutic protein efficacy. Selecting between prokaryotic and eukaryotic platforms depends on the target protein's complexity, expression yield requirements, and downstream application needs.
Genetic Engineering in Prokaryotes
Prokaryotic expression systems, such as Escherichia coli, offer rapid growth rates and high-yield protein production, making them ideal for genetic engineering applications. These systems enable efficient cloning and expression of recombinant DNA, facilitating large-scale synthesis of proteins, enzymes, and therapeutic agents. However, prokaryotes lack post-translational modifications common in eukaryotic cells, which can limit the functionality of some expressed proteins.
Genetic Engineering in Eukaryotes
Eukaryotic expression systems enable complex post-translational modifications essential for the functional expression of recombinant proteins in genetic engineering, unlike prokaryotic systems which lack these capabilities. Eukaryotic hosts such as yeast, insect, and mammalian cells facilitate accurate protein folding, glycosylation, and disulfide bond formation, critical for therapeutic protein production. The choice of expression system impacts protein yield, functionality, and suitability for downstream applications in biopharmaceutical development.
Post-Translational Modifications: Key Differences
Prokaryotic expression systems like Escherichia coli generally lack the machinery for complex post-translational modifications (PTMs) such as glycosylation, phosphorylation, and disulfide bond formation, limiting protein functionality and solubility. In contrast, eukaryotic expression systems, including yeast, insect, and mammalian cells, efficiently perform diverse PTMs necessary for proper protein folding, activity, and stability. These differences make eukaryotic systems more suitable for producing biologically active proteins requiring post-translational modifications critical for therapeutic and research applications.
Yield and Scalability Comparison
Prokaryotic expression systems, such as Escherichia coli, offer high protein yield and rapid scalability due to fast growth rates and simple culture requirements. Eukaryotic expression systems, including yeast, insect, and mammalian cells, generally produce lower yields but enable complex post-translational modifications essential for functional proteins. Scalability in eukaryotic systems is often limited by higher costs and longer culture times, making prokaryotic systems preferable for large-scale production when modifications are not critical.
Protein Folding and Solubility in Expression Hosts
Prokaryotic expression systems, such as Escherichia coli, often face challenges with protein folding, frequently resulting in inclusion bodies and low solubility of recombinant proteins. Eukaryotic expression hosts, including yeast and mammalian cells, provide more advanced post-translational modifications and chaperone-assisted folding mechanisms, enhancing the solubility and functional activity of complex proteins. Optimizing expression conditions in eukaryotic systems leads to higher yields of correctly folded proteins, essential for pharmaceutical and therapeutic applications.
Cost and Time Efficiency Analysis
Prokaryotic expression systems, such as Escherichia coli, offer significantly lower costs and faster protein production times compared to eukaryotic systems, making them ideal for rapid, large-scale protein synthesis. Eukaryotic expression systems, including yeast, insect, and mammalian cells, incur higher costs and longer culture durations due to complex post-translational modifications and growth requirements. The choice between prokaryotic and eukaryotic expression depends on balancing the urgency and budget constraints against the need for properly folded and functional proteins.
Applications in Therapeutic Protein Production
Prokaryotic expression systems, primarily using Escherichia coli, enable rapid and cost-effective production of therapeutic proteins such as insulin and growth factors but often lack proper post-translational modifications critical for protein functionality. Eukaryotic expression systems, including yeast, insect, and mammalian cells, provide essential post-translational modifications like glycosylation, enhancing protein stability, activity, and reducing immunogenicity in therapeutic applications. Selection between prokaryotic and eukaryotic expression platforms depends largely on the complexity of the target protein and the required functional modifications for clinical efficacy.
Choosing the Optimal Expression System
Choosing the optimal expression system in biotechnology depends on the specific requirements of the target protein, including post-translational modifications and yield. Prokaryotic expression systems like Escherichia coli offer rapid growth and high protein yield but lack complex post-translational modification capabilities. Eukaryotic expression systems such as yeast, insect, or mammalian cells provide proper folding and modifications essential for functional eukaryotic proteins, making them ideal for therapeutic protein production.
Prokaryotic Expression vs Eukaryotic Expression Infographic
 techiny.com
techiny.com