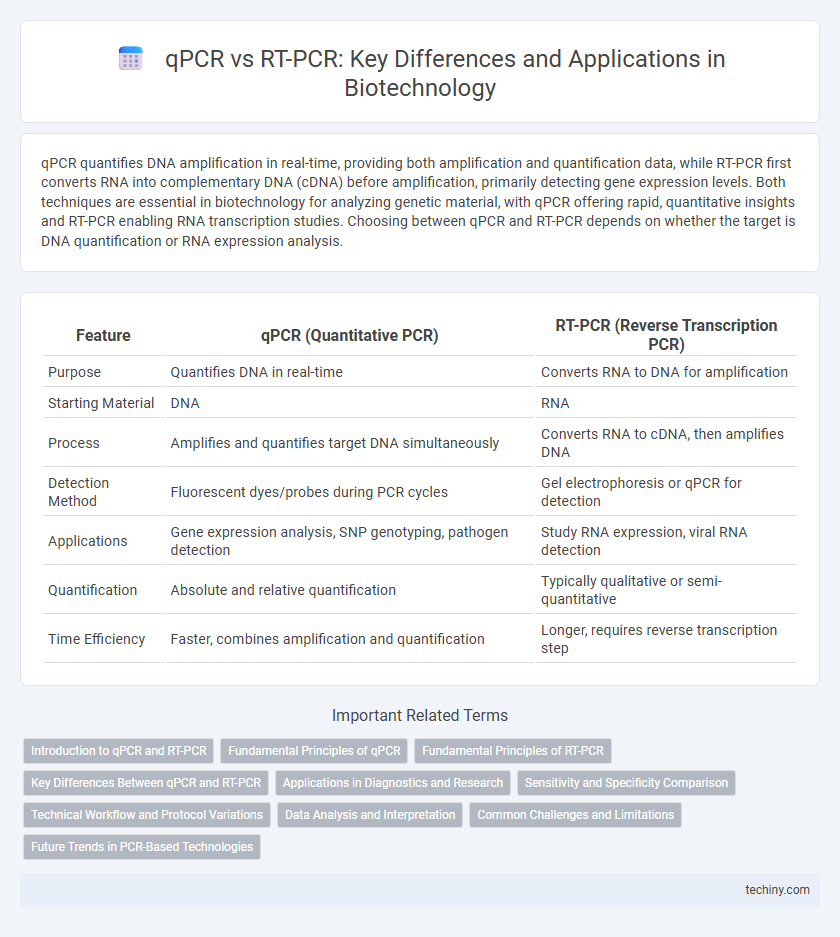
qPCR quantifies DNA amplification in real-time, providing both amplification and quantification data, while RT-PCR first converts RNA into complementary DNA (cDNA) before amplification, primarily detecting gene expression levels. Both techniques are essential in biotechnology for analyzing genetic material, with qPCR offering rapid, quantitative insights and RT-PCR enabling RNA transcription studies. Choosing between qPCR and RT-PCR depends on whether the target is DNA quantification or RNA expression analysis.
Table of Comparison
| Feature | qPCR (Quantitative PCR) | RT-PCR (Reverse Transcription PCR) |
|---|---|---|
| Purpose | Quantifies DNA in real-time | Converts RNA to DNA for amplification |
| Starting Material | DNA | RNA |
| Process | Amplifies and quantifies target DNA simultaneously | Converts RNA to cDNA, then amplifies DNA |
| Detection Method | Fluorescent dyes/probes during PCR cycles | Gel electrophoresis or qPCR for detection |
| Applications | Gene expression analysis, SNP genotyping, pathogen detection | Study RNA expression, viral RNA detection |
| Quantification | Absolute and relative quantification | Typically qualitative or semi-quantitative |
| Time Efficiency | Faster, combines amplification and quantification | Longer, requires reverse transcription step |
Introduction to qPCR and RT-PCR
qPCR (quantitative Polymerase Chain Reaction) quantifies DNA by amplifying target sequences in real-time, providing precise measurement of nucleic acid amounts during each cycle. RT-PCR (Reverse Transcription PCR) converts RNA into complementary DNA (cDNA) through reverse transcription before amplification, enabling gene expression analysis. Both techniques are essential for detecting genetic material, with qPCR offering quantitative data and RT-PCR allowing study of RNA transcripts.
Fundamental Principles of qPCR
qPCR, or quantitative polymerase chain reaction, quantifies DNA amplification in real-time by measuring fluorescent signals emitted during each cycle, enabling precise DNA copy number determination. This technique utilizes specific fluorescent dyes or probes that bind to the target DNA, producing fluorescence proportional to the amount of amplified DNA. Unlike RT-PCR, which converts RNA into cDNA before amplification, qPCR emphasizes continuous monitoring of amplification, providing quantitative data essential for gene expression analysis and molecular diagnostics.
Fundamental Principles of RT-PCR
RT-PCR, or reverse transcription polymerase chain reaction, involves converting RNA into complementary DNA (cDNA) using reverse transcriptase before amplification, enabling detection of gene expression. This method targets RNA sequences, distinguishing it from qPCR, which quantifies DNA amplification in real-time. The fundamental principle of RT-PCR lies in its ability to analyze RNA targets through enzymatic reverse transcription followed by PCR amplification for precise genetic study.
Key Differences Between qPCR and RT-PCR
qPCR (quantitative PCR) quantifies DNA amplification by measuring fluorescence in real-time, allowing precise quantification of nucleic acids. RT-PCR (reverse transcription PCR) converts RNA into complementary DNA (cDNA) before amplification, enabling analysis of gene expression from RNA samples. Key differences include qPCR's real-time quantification capability versus RT-PCR's initial RNA-to-DNA conversion step, with qPCR often used for gene expression quantification and RT-PCR for detecting RNA presence.
Applications in Diagnostics and Research
qPCR enables precise quantification of nucleic acids, facilitating the measurement of gene expression levels and viral load in clinical diagnostics and molecular research. RT-PCR converts RNA into complementary DNA, allowing detection of RNA viruses and gene expression analysis, crucial for infectious disease diagnosis and transcriptomics studies. Both technologies drive advancements in personalized medicine, oncology, and infectious disease monitoring by providing sensitive and specific detection of genetic material.
Sensitivity and Specificity Comparison
qPCR demonstrates higher sensitivity than RT-PCR by quantifying nucleic acids in real-time, allowing detection of low template concentrations with precise fluorescence measurements. Specificity in qPCR is enhanced by the use of sequence-specific probes, reducing non-specific amplification compared to conventional RT-PCR, which relies on endpoint analysis. This combination of sensitive detection and probe-based specificity makes qPCR the preferred method for accurate quantification of gene expression and pathogen detection in biotechnology.
Technical Workflow and Protocol Variations
qPCR quantifies DNA amplification in real-time using fluorescent dyes or probes, enabling precise quantification during each cycle, whereas RT-PCR converts RNA to cDNA before amplification and typically assesses endpoints post-amplification. The technical workflow of qPCR integrates reverse transcription and amplification in a single step (one-step RT-qPCR) or separates them into distinct stages (two-step RT-qPCR), affecting sensitivity and specificity. Protocol variations include differences in primer design, reverse transcriptase enzymes, and thermal cycling conditions, which influence efficiency, quantification accuracy, and detection limits across diverse sample types.
Data Analysis and Interpretation
qPCR enables real-time quantification of nucleic acids by measuring fluorescence intensity during amplification cycles, providing dynamic data analysis through threshold cycle (Ct) values for precise gene expression quantification. RT-PCR converts RNA into complementary DNA before amplification but typically yields qualitative or semi-quantitative data, limiting detailed expression analysis. Advanced qPCR data interpretation involves normalization with reference genes and calculation of fold changes using methods like DDCt, ensuring accurate and reproducible results in gene expression studies.
Common Challenges and Limitations
qPCR and RT-PCR face common challenges such as primer-dimer formation, nonspecific amplification, and contamination risks that can compromise result accuracy. Both techniques require rigorous optimization of reaction conditions and precise control of RNA quality to ensure reproducibility. Limitations include the need for high-quality templates and potential inhibition by sample contaminants, affecting amplification efficiency and quantification reliability.
Future Trends in PCR-Based Technologies
Emerging trends in PCR-based technologies emphasize enhanced sensitivity and automation, with digital PCR and multiplex qPCR playing pivotal roles in diagnostics and personalized medicine. Integrating artificial intelligence algorithms with qPCR and RT-PCR data accelerates interpretation accuracy and streamlines workflow efficiency. The convergence of microfluidics and PCR platforms is driving miniaturization and point-of-care testing advancements, expanding accessibility in clinical and environmental biotechnology applications.
qPCR vs RT-PCR Infographic
 techiny.com
techiny.com