Restriction enzymes and CRISPR nucleases are essential tools in biotechnology for precise DNA manipulation in pet-related genetic research. Restriction enzymes recognize specific short DNA sequences and cut at fixed sites, making them valuable for cloning and analysis, whereas CRISPR nucleases offer targeted genome editing with high precision by using guide RNA to locate specific genetic regions. CRISPR technology enables more versatile and efficient modifications in pet genetics, such as gene correction and functional studies, surpassing the traditional limitations of restriction enzymes.
Table of Comparison
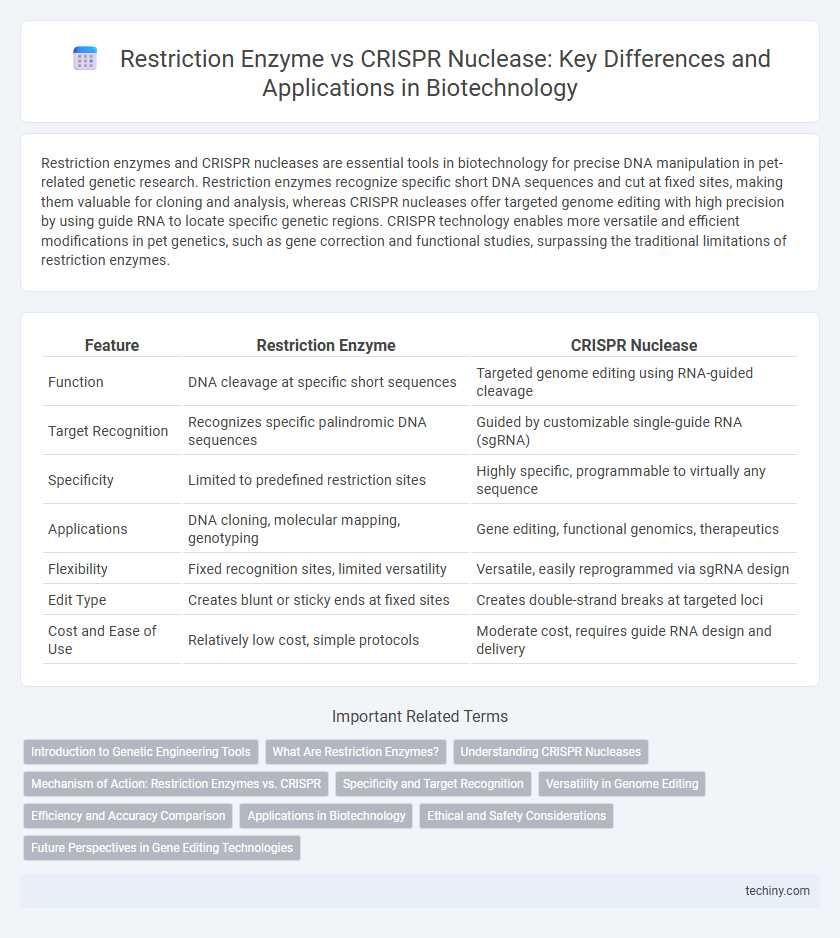
| Feature | Restriction Enzyme | CRISPR Nuclease |
|---|---|---|
| Function | DNA cleavage at specific short sequences | Targeted genome editing using RNA-guided cleavage |
| Target Recognition | Recognizes specific palindromic DNA sequences | Guided by customizable single-guide RNA (sgRNA) |
| Specificity | Limited to predefined restriction sites | Highly specific, programmable to virtually any sequence |
| Applications | DNA cloning, molecular mapping, genotyping | Gene editing, functional genomics, therapeutics |
| Flexibility | Fixed recognition sites, limited versatility | Versatile, easily reprogrammed via sgRNA design |
| Edit Type | Creates blunt or sticky ends at fixed sites | Creates double-strand breaks at targeted loci |
| Cost and Ease of Use | Relatively low cost, simple protocols | Moderate cost, requires guide RNA design and delivery |
Introduction to Genetic Engineering Tools
Restriction enzymes are naturally occurring proteins that recognize specific DNA sequences and cut molecular strands at these sites, enabling gene cloning and molecular analysis. CRISPR nucleases, derived from bacterial adaptive immune systems, allow precise genome editing by utilizing a guide RNA to target and cleave specific DNA sequences, offering greater flexibility and efficiency. Both tools revolutionize genetic engineering by enabling targeted modifications, but CRISPR systems provide enhanced specificity and ease of customization compared to traditional restriction enzymes.
What Are Restriction Enzymes?
Restriction enzymes, also known as restriction endonucleases, are proteins that recognize specific nucleotide sequences within DNA and cut the DNA at or near these sites, enabling precise genetic manipulation. These enzymes serve as essential tools in molecular cloning, genetic mapping, and recombinant DNA technology by generating reproducible DNA fragments. Restriction enzymes differ from CRISPR nucleases, which use RNA-guided mechanisms to target DNA, providing greater flexibility and specificity in genome editing applications.
Understanding CRISPR Nucleases
CRISPR nucleases, such as Cas9, offer precise genome editing by targeting specific DNA sequences guided by RNA molecules, contrasting with restriction enzymes that recognize fixed DNA motifs. The programmable nature of CRISPR nucleases enables efficient and versatile manipulation of genetic material across various organisms. This technology has revolutionized biotechnology by allowing customizable gene editing with high specificity and minimal off-target effects.
Mechanism of Action: Restriction Enzymes vs. CRISPR
Restriction enzymes cleave DNA at specific nucleotide sequences by recognizing palindromic sites and cutting both DNA strands, resulting in predictable fragment patterns. CRISPR nucleases, guided by RNA sequences complementary to target DNA, induce double-strand breaks at precise genomic locations, allowing for targeted gene editing. Unlike restriction enzymes, CRISPR systems provide programmable specificity through guide RNA, enabling versatile manipulation of genetic material.
Specificity and Target Recognition
Restriction enzymes recognize specific DNA sequences, typically 4-8 base pairs long, and cleave at or near these sites, providing high specificity but limited to predefined recognition patterns. CRISPR nucleases, guided by customizable RNA sequences, enable precise targeting of virtually any genomic locus, offering greater versatility and specificity in gene editing applications. The programmable nature of CRISPR significantly enhances target recognition beyond the fixed sequence constraints inherent to restriction enzymes.
Versatility in Genome Editing
Restriction enzymes recognize specific DNA sequences and cut at fixed sites, providing precise but limited targeting capabilities in genome editing. CRISPR nucleases utilize RNA-guided mechanisms to target virtually any DNA sequence, offering unparalleled versatility across diverse genomes. The programmability and adaptability of CRISPR systems enable efficient, multiplexed modifications beyond the scope of traditional restriction enzymes.
Efficiency and Accuracy Comparison
Restriction enzymes exhibit high specificity for their palindromic DNA sequences but are limited by fixed recognition sites, reducing flexibility in genome editing efficiency. CRISPR nucleases, particularly Cas9, demonstrate superior efficiency and accuracy due to programmable RNA guides enabling precise targeting across diverse genomic loci. Off-target effects in CRISPR systems can be minimized with engineered variants, making them more adaptable and effective than restriction enzymes for complex genetic modifications.
Applications in Biotechnology
Restriction enzymes enable precise DNA cutting at specific sequences, facilitating cloning, gene mapping, and DNA fingerprinting in biotechnology. CRISPR nucleases provide programmable, efficient genome editing with higher specificity, revolutionizing gene therapy, functional genomics, and crop improvement. These tools complement each other, with restriction enzymes preferred for traditional molecular cloning and CRISPR nucleases driving advanced genome engineering applications.
Ethical and Safety Considerations
Restriction enzymes have long been used as precise molecular scissors in genetic engineering with a well-established safety profile, whereas CRISPR nucleases offer enhanced efficiency and versatility but raise complex ethical concerns regarding off-target effects and potential germline modifications. The use of CRISPR technology necessitates stringent regulatory oversight to mitigate risks such as unintended genetic alterations and ecological impacts. Balancing innovation with ethical responsibility requires continuous evaluation of both technologies' implications on human health and biodiversity.
Future Perspectives in Gene Editing Technologies
Restriction enzymes, historically pivotal in gene editing by recognizing specific DNA sequences, face limitations in precision and target scope compared to CRISPR nucleases, which offer programmable specificity and versatility in editing genomes across diverse organisms. Advancements in CRISPR technology are driving next-generation gene therapies, agricultural improvements, and synthetic biology applications, emphasizing increased accuracy and reduced off-target effects. Emerging developments in base editing and prime editing, building on CRISPR systems, promise transformative impacts on personalized medicine and functional genomics by enabling precise, efficient, and customizable genetic modifications.
Restriction Enzyme vs CRISPR Nuclease Infographic
 techiny.com
techiny.com