Single nucleotide polymorphisms (SNPs) are variations at a single base pair in the DNA sequence, influencing traits and disease susceptibility in pets by altering gene function or expression. Copy number variations (CNVs) involve larger segments of DNA that are duplicated or deleted, significantly impacting gene dosage and phenotypic diversity. Understanding the interplay between SNPs and CNVs enhances precision in pet biotechnology, aiding in the development of targeted genetic therapies and personalized veterinary care.
Table of Comparison
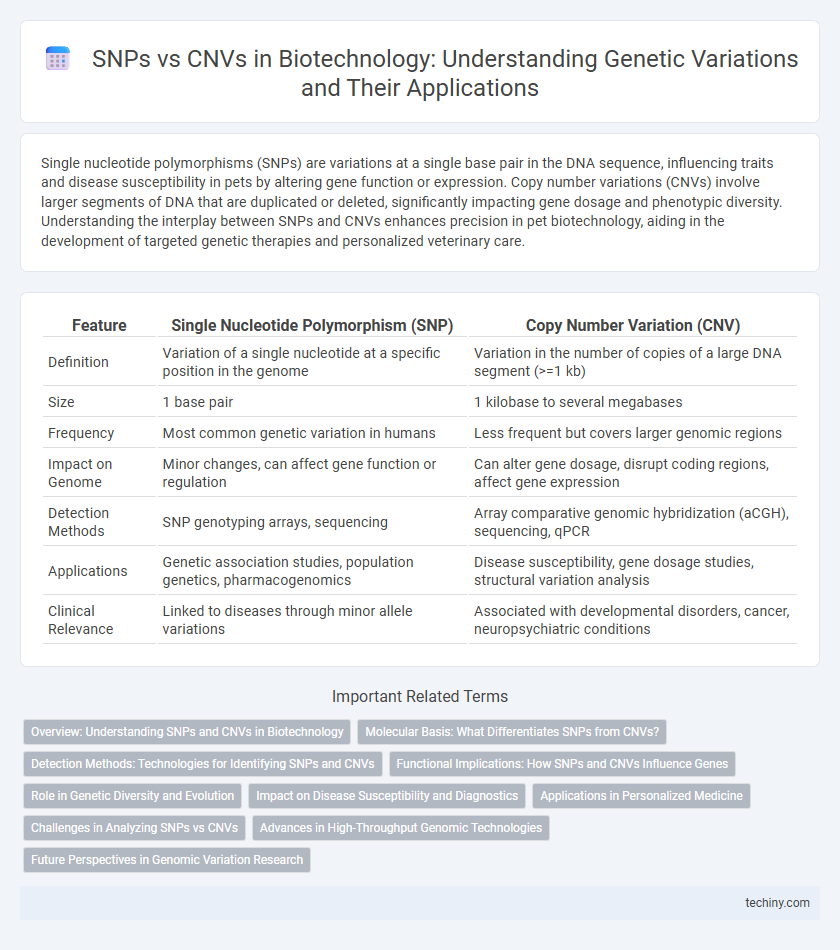
| Feature | Single Nucleotide Polymorphism (SNP) | Copy Number Variation (CNV) |
|---|---|---|
| Definition | Variation of a single nucleotide at a specific position in the genome | Variation in the number of copies of a large DNA segment (>=1 kb) |
| Size | 1 base pair | 1 kilobase to several megabases |
| Frequency | Most common genetic variation in humans | Less frequent but covers larger genomic regions |
| Impact on Genome | Minor changes, can affect gene function or regulation | Can alter gene dosage, disrupt coding regions, affect gene expression |
| Detection Methods | SNP genotyping arrays, sequencing | Array comparative genomic hybridization (aCGH), sequencing, qPCR |
| Applications | Genetic association studies, population genetics, pharmacogenomics | Disease susceptibility, gene dosage studies, structural variation analysis |
| Clinical Relevance | Linked to diseases through minor allele variations | Associated with developmental disorders, cancer, neuropsychiatric conditions |
Overview: Understanding SNPs and CNVs in Biotechnology
Single nucleotide polymorphisms (SNPs) represent variations at a single base pair in DNA, serving as crucial genetic markers for identifying hereditary traits and disease susceptibility in biotechnology research. Copy number variations (CNVs) involve larger segments of the genome that are duplicated or deleted, impacting gene dosage and contributing to phenotypic diversity and complex disorders. Both SNPs and CNVs are fundamental for precision medicine, enabling advanced genetic mapping, biomarker discovery, and the development of targeted therapeutic interventions.
Molecular Basis: What Differentiates SNPs from CNVs?
Single nucleotide polymorphisms (SNPs) are variations at a single base pair in the DNA sequence, affecting one nucleotide, whereas copy number variations (CNVs) involve large segments of the genome that are duplicated or deleted, resulting in variable copy numbers of one or more genes. SNPs typically represent point mutations occurring at specific loci, while CNVs reflect structural genomic alterations that can span thousands to millions of base pairs. The molecular basis of these variations influences gene expression and phenotypic diversity differently due to their scale and genomic impact.
Detection Methods: Technologies for Identifying SNPs and CNVs
Detection methods for Single Nucleotide Polymorphisms (SNPs) primarily include high-throughput genotyping arrays and next-generation sequencing (NGS), enabling precise identification of single base changes across genomes. Copy Number Variations (CNVs) are often detected using comparative genomic hybridization (CGH) arrays, quantitative PCR (qPCR), or NGS-based read depth analysis to quantify genomic segment duplication or deletion. Emerging techniques such as digital droplet PCR and long-read sequencing enhance resolution and accuracy for both SNP and CNV detection in complex genomic regions.
Functional Implications: How SNPs and CNVs Influence Genes
Single nucleotide polymorphisms (SNPs) affect gene function by altering amino acid sequences or regulatory elements, potentially impacting protein structure and gene expression. Copy number variations (CNVs) influence gene dosage, leading to increased or decreased gene product levels and affecting phenotypic traits. Both SNPs and CNVs play critical roles in genetic diversity and disease susceptibility by modifying gene function through distinct molecular mechanisms.
Role in Genetic Diversity and Evolution
Single nucleotide polymorphisms (SNPs) represent single base pair changes in the genome and serve as crucial markers for tracing genetic diversity among populations due to their high frequency and stability. Copy number variations (CNVs), involving larger segments of DNA that are duplicated or deleted, significantly contribute to phenotypic diversity and adaptive evolution by altering gene dosage and expression levels. Both SNPs and CNVs drive evolutionary processes by generating genetic variation that natural selection can act upon, but CNVs often have more pronounced effects on complex traits and disease susceptibility.
Impact on Disease Susceptibility and Diagnostics
Single nucleotide polymorphisms (SNPs) and copy number variations (CNVs) each contribute uniquely to disease susceptibility and diagnostic precision. SNPs, involving a single base alteration, can influence gene function and regulate disease-associated traits, making them critical markers for genome-wide association studies (GWAS) in complex diseases like diabetes and cancer. CNVs, characterized by variations in the number of copies of large DNA segments, can lead to gene dosage imbalances and are implicated in neurological disorders and immune system dysfunctions, thus offering diagnostic insights into conditions that involve large-scale genomic changes.
Applications in Personalized Medicine
Single nucleotide polymorphisms (SNPs) serve as precise biomarkers for identifying individual genetic variations that influence drug metabolism and disease susceptibility, enabling tailored therapeutic strategies in personalized medicine. Copy number variations (CNVs) contribute to phenotypic diversity by altering gene dosage, impacting gene expression levels, and offering critical insights for diagnosing complex genetic disorders and optimizing treatment regimens. Integrating SNP and CNV data enhances the accuracy of pharmacogenomic profiles, fostering more effective, patient-specific interventions and advancing precision healthcare outcomes.
Challenges in Analyzing SNPs vs CNVs
Analyzing single nucleotide polymorphisms (SNPs) presents challenges such as detecting low-frequency variants and distinguishing true polymorphisms from sequencing errors, requiring high-throughput sequencing accuracy and advanced bioinformatics tools. Copy number variation (CNV) analysis is complicated by the variability in CNV size, genomic context, and breakpoints, demanding specialized algorithms to accurately quantify and map these structural alterations. Both SNP and CNV analysis face difficulties in interpreting functional impacts and integrating data for comprehensive genomic insights in complex disorders.
Advances in High-Throughput Genomic Technologies
High-throughput genomic technologies have revolutionized the detection and analysis of single nucleotide polymorphisms (SNPs) and copy number variations (CNVs), enabling more precise genome-wide association studies and personalized medicine applications. Next-generation sequencing (NGS) platforms and microarray-based methods provide comprehensive coverage and high resolution, allowing researchers to identify millions of SNPs and large-scale CNVs with improved accuracy and throughput. Advances in bioinformatics tools further enhance the interpretation of complex genomic data, facilitating novel insights into genetic variation and disease susceptibility.
Future Perspectives in Genomic Variation Research
Future research in genomic variation will intensify the comparative analysis of Single Nucleotide Polymorphisms (SNPs) and Copy Number Variations (CNVs) to enhance precision medicine and disease association studies. Advancements in high-throughput sequencing technologies and machine learning algorithms will enable more comprehensive identification and functional characterization of SNPs and CNVs across diverse populations. Integrating multi-omics data and large-scale biobank resources is expected to uncover novel genotype-phenotype correlations, advancing personalized therapeutic strategies and genomic medicine innovations.
Single nucleotide polymorphism (SNP) vs Copy number variation (CNV) Infographic
 techiny.com
techiny.com