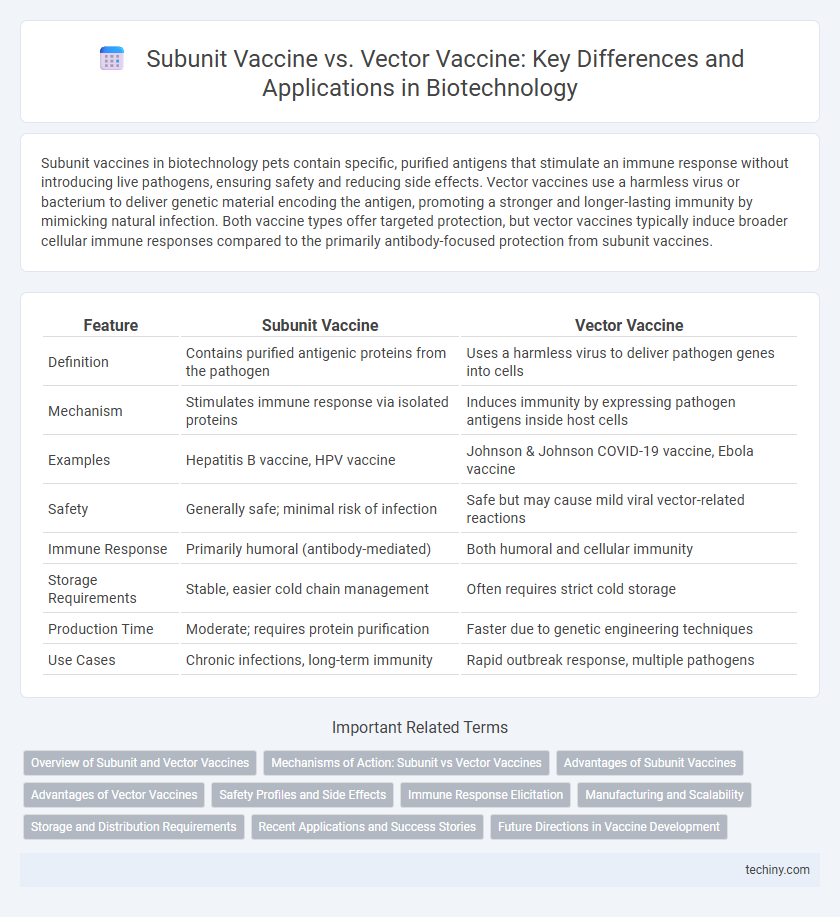
Subunit vaccines in biotechnology pets contain specific, purified antigens that stimulate an immune response without introducing live pathogens, ensuring safety and reducing side effects. Vector vaccines use a harmless virus or bacterium to deliver genetic material encoding the antigen, promoting a stronger and longer-lasting immunity by mimicking natural infection. Both vaccine types offer targeted protection, but vector vaccines typically induce broader cellular immune responses compared to the primarily antibody-focused protection from subunit vaccines.
Table of Comparison
| Feature | Subunit Vaccine | Vector Vaccine |
|---|---|---|
| Definition | Contains purified antigenic proteins from the pathogen | Uses a harmless virus to deliver pathogen genes into cells |
| Mechanism | Stimulates immune response via isolated proteins | Induces immunity by expressing pathogen antigens inside host cells |
| Examples | Hepatitis B vaccine, HPV vaccine | Johnson & Johnson COVID-19 vaccine, Ebola vaccine |
| Safety | Generally safe; minimal risk of infection | Safe but may cause mild viral vector-related reactions |
| Immune Response | Primarily humoral (antibody-mediated) | Both humoral and cellular immunity |
| Storage Requirements | Stable, easier cold chain management | Often requires strict cold storage |
| Production Time | Moderate; requires protein purification | Faster due to genetic engineering techniques |
| Use Cases | Chronic infections, long-term immunity | Rapid outbreak response, multiple pathogens |
Overview of Subunit and Vector Vaccines
Subunit vaccines contain purified pieces of the pathogen, such as proteins or polysaccharides, to stimulate a specific immune response without introducing live components, enhancing safety and stability. Vector vaccines utilize a harmless virus or bacterium to deliver genetic material encoding an antigen, prompting the host cells to produce the antigen and trigger immunity. Both vaccine types are integral in biotechnology for designing targeted immunizations with distinct mechanisms and applications.
Mechanisms of Action: Subunit vs Vector Vaccines
Subunit vaccines deliver specific purified antigens, such as proteins or peptides, to stimulate an immune response without introducing live components, primarily activating B cells and antibody production. Vector vaccines use a harmless viral vector to transport genetic material encoding the target antigen into host cells, triggering both humoral and cellular immunity by promoting antigen presentation via MHC class I and II pathways. The distinct mechanisms influence vaccine efficacy, durability, and safety profiles in various infectious diseases.
Advantages of Subunit Vaccines
Subunit vaccines offer enhanced safety profiles by containing only specific antigenic components, minimizing the risk of adverse reactions compared to vector vaccines that use live vectors. These vaccines provide targeted immune responses with reduced likelihood of vector-related immunity interference, ensuring consistent efficacy across diverse populations. Their stability and ease of manufacturing facilitate widespread distribution and storage, especially important in global immunization efforts.
Advantages of Vector Vaccines
Vector vaccines provide robust cellular and humoral immune responses by delivering antigen genes directly into host cells, enhancing the body's ability to recognize and combat pathogens. They often induce longer-lasting immunity compared to subunit vaccines, which rely solely on isolated protein antigens. The use of viral vectors in these vaccines allows for efficient antigen presentation and potential for broader protection against diverse strains.
Safety Profiles and Side Effects
Subunit vaccines contain purified antigenic proteins, minimizing adverse immune reactions and offering an excellent safety profile with rare side effects such as mild injection site reactions and low systemic responses. Vector vaccines use modified viruses to deliver genetic material, potentially causing stronger immune responses but also rarely inducing mild to moderate side effects like fever, fatigue, and injection site soreness. The non-replicating viral vectors in vector vaccines are engineered for safety, yet subunit vaccines exhibit fewer risks of systemic inflammation, making them preferable for immunocompromised individuals.
Immune Response Elicitation
Subunit vaccines stimulate immune response by introducing specific protein antigens that directly activate B cells and helper T cells without using live components, minimizing safety risks. Vector vaccines deliver genetic material encoding antigens via modified viral vectors, inducing robust cellular and humoral immunity through endogenous antigen expression and presentation. The distinct mechanisms influence the strength and breadth of the immune response, with vector vaccines typically eliciting stronger cytotoxic T lymphocyte activation compared to subunit vaccines.
Manufacturing and Scalability
Subunit vaccines are produced by isolating specific proteins from a pathogen, enabling precise control over antigen composition and ensuring high purity, which simplifies quality control during manufacturing. Vector vaccines utilize recombinant viruses to deliver genetic material encoding antigens, requiring complex cell culture systems and biosafety measures, often resulting in longer production timelines. Scalability of subunit vaccines benefits from established fermentation and purification processes, whereas vector vaccines face challenges in large-scale viral vector production and maintaining consistent vector potency.
Storage and Distribution Requirements
Subunit vaccines typically require refrigeration at 2-8degC, making their storage and distribution easier in standard cold chain systems, whereas vector vaccines often demand stricter temperature controls, sometimes requiring ultra-cold storage at -70degC. The stability of subunit vaccines reduces logistical challenges, enabling broader distribution in regions with limited cold chain infrastructure. Vector vaccines, due to their temperature sensitivity, necessitate advanced cold chain technology to maintain efficacy during transport and storage.
Recent Applications and Success Stories
Subunit vaccines, such as the HPV vaccine Cervarix, have demonstrated success by using purified antigenic components to elicit targeted immune responses with minimal side effects. Vector vaccines, exemplified by the Oxford-AstraZeneca COVID-19 vaccine, utilize viral vectors like adenoviruses to deliver genetic material and induce robust cellular and humoral immunity. Recent applications include effective pandemic responses and cancer immunotherapies, highlighting their complementary roles in precision medicine and infectious disease control.
Future Directions in Vaccine Development
Subunit vaccines offer targeted immune responses by using specific antigens, while vector vaccines employ modified viruses to deliver genetic material, enabling broader cellular immunity. Future directions emphasize combining the precision of subunit vaccines with the robust immunogenicity of vector platforms, leveraging mRNA technology and novel adjuvants to enhance efficacy and durability. Advances in synthetic biology and nanoparticle delivery systems aim to optimize vaccine stability, reduce side effects, and accelerate rapid response against emerging pathogens.
**Subunit vaccine vs Vector vaccine** Infographic
 techiny.com
techiny.com