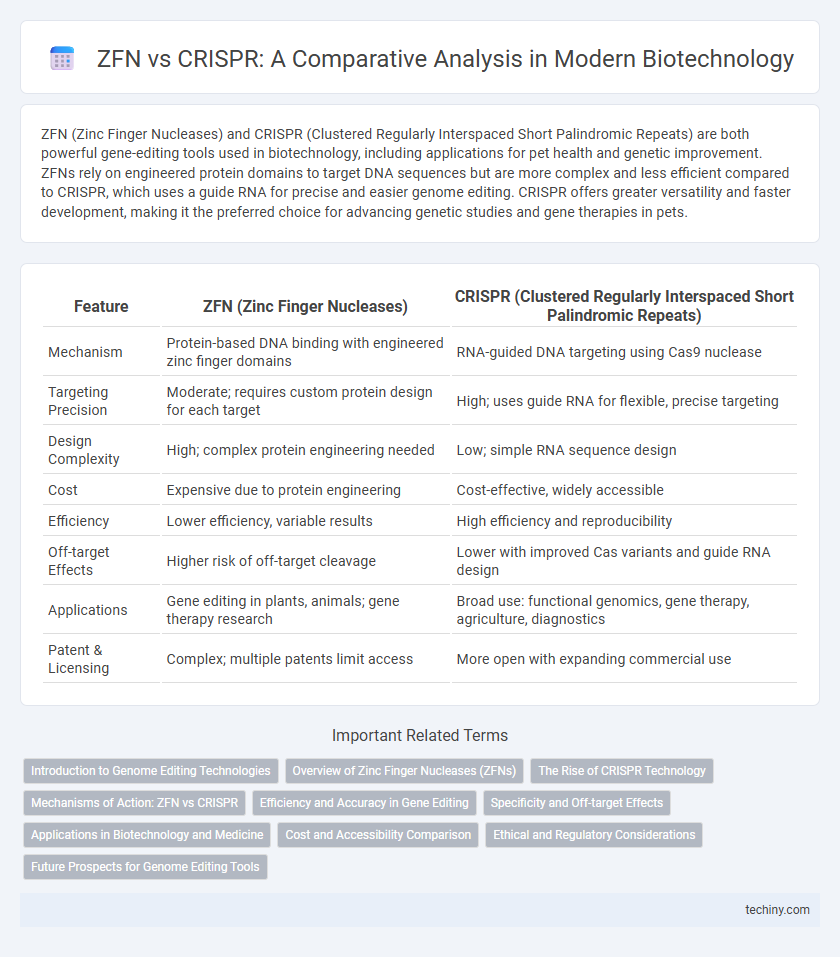
ZFN (Zinc Finger Nucleases) and CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) are both powerful gene-editing tools used in biotechnology, including applications for pet health and genetic improvement. ZFNs rely on engineered protein domains to target DNA sequences but are more complex and less efficient compared to CRISPR, which uses a guide RNA for precise and easier genome editing. CRISPR offers greater versatility and faster development, making it the preferred choice for advancing genetic studies and gene therapies in pets.
Table of Comparison
| Feature | ZFN (Zinc Finger Nucleases) | CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) |
|---|---|---|
| Mechanism | Protein-based DNA binding with engineered zinc finger domains | RNA-guided DNA targeting using Cas9 nuclease |
| Targeting Precision | Moderate; requires custom protein design for each target | High; uses guide RNA for flexible, precise targeting |
| Design Complexity | High; complex protein engineering needed | Low; simple RNA sequence design |
| Cost | Expensive due to protein engineering | Cost-effective, widely accessible |
| Efficiency | Lower efficiency, variable results | High efficiency and reproducibility |
| Off-target Effects | Higher risk of off-target cleavage | Lower with improved Cas variants and guide RNA design |
| Applications | Gene editing in plants, animals; gene therapy research | Broad use: functional genomics, gene therapy, agriculture, diagnostics |
| Patent & Licensing | Complex; multiple patents limit access | More open with expanding commercial use |
Introduction to Genome Editing Technologies
Genome editing technologies such as Zinc Finger Nucleases (ZFNs) and CRISPR-Cas9 enable precise modifications to the DNA sequence within living cells. ZFNs utilize engineered protein domains to target specific DNA sequences, whereas CRISPR-Cas9 employs guide RNA for sequence recognition, offering greater flexibility and efficiency. These tools have revolutionized biotechnology, facilitating advancements in gene therapy, agriculture, and functional genomics by allowing targeted gene disruption, correction, or insertion.
Overview of Zinc Finger Nucleases (ZFNs)
Zinc Finger Nucleases (ZFNs) are engineered DNA-binding proteins that facilitate targeted genome editing by creating double-strand breaks at specific DNA sequences. Composed of zinc finger domains fused to a FokI nuclease, ZFNs enable precise gene modifications through the cell's natural repair mechanisms, primarily non-homologous end joining or homologous recombination. Although ZFNs offer high specificity, their complex design and engineering compared to CRISPR have limited widespread adoption in genetic research and therapeutic applications.
The Rise of CRISPR Technology
CRISPR technology has revolutionized gene editing by offering greater precision, efficiency, and ease of use compared to Zinc Finger Nucleases (ZFNs). Unlike ZFNs, which require complex protein engineering for each target, CRISPR utilizes a simple RNA guide that can be easily programmed to target virtually any genetic sequence. This adaptability and scalability have accelerated advancements in functional genomics, therapeutic development, and agricultural biotechnology.
Mechanisms of Action: ZFN vs CRISPR
Zinc Finger Nucleases (ZFNs) utilize engineered zinc finger proteins to recognize specific DNA sequences, paired with the FokI nuclease domain to induce double-strand breaks at targeted sites. CRISPR-Cas9 employs a guide RNA to direct the Cas9 nuclease to complementary DNA sequences, enabling precise genome editing by creating double-stranded breaks. Unlike ZFNs, CRISPR allows easier customization and multiplexing, enhancing its efficiency in targeted gene modification.
Efficiency and Accuracy in Gene Editing
Zinc Finger Nucleases (ZFNs) and CRISPR systems both enable precise gene editing, but CRISPR-Cas9 offers higher efficiency by targeting multiple sites simultaneously with greater ease of design. ZFNs rely on protein-DNA interactions, which can reduce accuracy due to off-target effects from complex protein engineering requirements. CRISPR's RNA-guided mechanism enhances specificity, minimizing unintended mutations and making it the preferred choice for efficient and accurate genome modification in biotechnology.
Specificity and Off-target Effects
Zinc Finger Nucleases (ZFNs) exhibit high target specificity due to engineered zinc finger domains recognizing precise DNA sequences, yet their design complexity can limit widespread use. CRISPR-Cas9 offers simpler programming through RNA-guided targeting, but it presents higher risks of off-target effects causing unintended genetic modifications. Advances in engineered Cas9 variants and improved guide RNA designs are enhancing CRISPR specificity, reducing off-target activity for safer genome editing applications.
Applications in Biotechnology and Medicine
Zinc Finger Nucleases (ZFNs) enable precise genome editing used in gene therapy for monogenic disorders and crop improvement, while CRISPR-Cas9 offers higher efficiency and multiplexing capability for diverse applications such as cancer immunotherapy and disease modeling. CRISPR's ease of design accelerates development of genetic modifications in pharmaceutical research and regenerative medicine. Both tools advance targeted genetic interventions, but CRISPR's versatility has expanded its utility across therapeutic gene editing and agricultural biotechnology.
Cost and Accessibility Comparison
Zinc Finger Nucleases (ZFNs) are significantly more expensive and complex to engineer compared to CRISPR-Cas9 systems, limiting their accessibility to large institutions with substantial funding. In contrast, CRISPR technology offers a lower-cost, user-friendly alternative with widespread availability, making gene editing accessible to academic labs and smaller biotech startups. The affordability and simplicity of CRISPR have driven its rapid adoption in biotechnological research and therapeutic development.
Ethical and Regulatory Considerations
ZFN (Zinc Finger Nucleases) and CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) each present distinct ethical and regulatory challenges in biotechnology due to their gene-editing precision and potential off-target effects. Regulatory frameworks must address concerns about germline modifications, informed consent, and potential ecological impacts, with CRISPR's relative ease of use prompting more urgent policy discussions worldwide. Ethical debates emphasize equitable access, potential for unintended consequences, and the need for international collaboration to establish guidelines that balance innovation with safety.
Future Prospects for Genome Editing Tools
Zinc Finger Nucleases (ZFNs) offer precise genome editing through customizable DNA-binding domains but face challenges in design complexity and off-target effects. CRISPR-Cas systems provide greater versatility, efficiency, and easier programmability, accelerating therapeutic advancements and agricultural improvements. Future prospects emphasize enhanced specificity, reduced off-target impacts, and integration with base and prime editing technologies to revolutionize precision medicine and functional genomics.
ZFN vs CRISPR Infographic
 techiny.com
techiny.com