Biosimilars are biologic products highly similar to an original biologic medicine but made using living organisms, reflecting complex molecular structures and manufacturing processes. Generics are identical copies of small-molecule drugs with simpler chemical structures, manufactured through standardized chemical synthesis. Understanding the distinctions between biosimilars and generics is crucial for advancing biotechnological innovations in pet healthcare and ensuring effective therapeutic options.
Table of Comparison
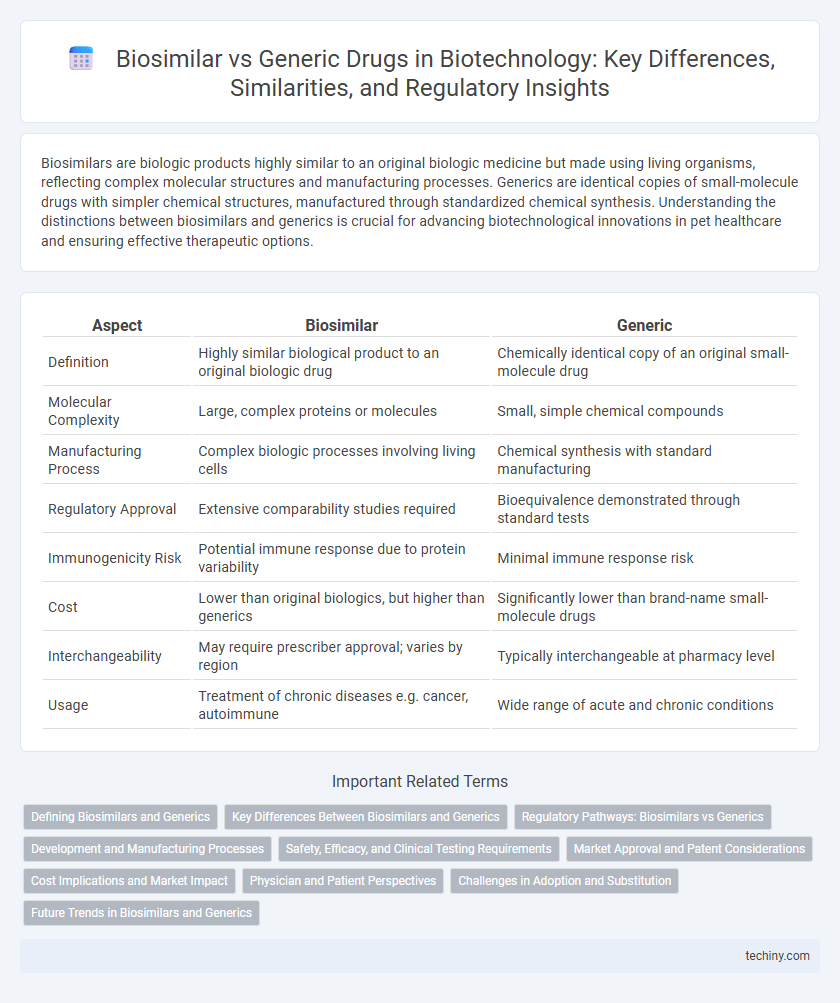
| Aspect | Biosimilar | Generic |
|---|---|---|
| Definition | Highly similar biological product to an original biologic drug | Chemically identical copy of an original small-molecule drug |
| Molecular Complexity | Large, complex proteins or molecules | Small, simple chemical compounds |
| Manufacturing Process | Complex biologic processes involving living cells | Chemical synthesis with standard manufacturing |
| Regulatory Approval | Extensive comparability studies required | Bioequivalence demonstrated through standard tests |
| Immunogenicity Risk | Potential immune response due to protein variability | Minimal immune response risk |
| Cost | Lower than original biologics, but higher than generics | Significantly lower than brand-name small-molecule drugs |
| Interchangeability | May require prescriber approval; varies by region | Typically interchangeable at pharmacy level |
| Usage | Treatment of chronic diseases e.g. cancer, autoimmune | Wide range of acute and chronic conditions |
Defining Biosimilars and Generics
Biosimilars are complex biologic products highly similar to an original licensed biologic, demonstrating no clinically meaningful differences in safety, purity, and potency. Generics are chemically synthesized, identical copies of small-molecule drugs with identical active ingredients, strength, and dosage forms. The regulatory pathways for biosimilars involve extensive analytical, non-clinical, and clinical data, whereas generics require proof of bioequivalence to the reference product.
Key Differences Between Biosimilars and Generics
Biosimilars are complex biologic products derived from living organisms, designed to be highly similar but not identical to an original biologic drug, while generics are identical chemical copies of small-molecule drugs. Biosimilars require extensive clinical trials to demonstrate similarity in safety, efficacy, and immunogenicity, whereas generics primarily need to prove bioequivalence through pharmacokinetic studies. Manufacturing biosimilars involves sophisticated biotechnological processes with variability risks, contrasting with the simpler chemical synthesis and replication methods used for generics.
Regulatory Pathways: Biosimilars vs Generics
Regulatory pathways for biosimilars involve rigorous comparability exercises to demonstrate similarity to the reference biologic in terms of safety, efficacy, and quality, differing significantly from generics that require bioequivalence studies with small-molecule drugs. Biosimilar approval processes, governed by agencies like the FDA and EMA, demand extensive analytical characterization, clinical trials, and post-market pharmacovigilance, whereas generic drugs primarily rely on demonstrating identical active ingredients and pharmaceutical equivalence. These distinct regulatory frameworks reflect the complexity of biologics compared to chemically synthesized drugs, influencing development timelines and market entry strategies.
Development and Manufacturing Processes
Biosimilars undergo complex development processes involving extensive characterization and clinical trials to demonstrate similarity to the reference biologic, unlike generics which rely on identical chemical synthesis. Manufacturing biosimilars requires sophisticated biotechnological methods with living cells, stringent process controls, and batch-to-batch consistency to ensure efficacy and safety. Generic drug production typically uses standardized chemical synthesis with more straightforward quality control protocols, resulting in faster and less costly development cycles.
Safety, Efficacy, and Clinical Testing Requirements
Biosimilars undergo rigorous analytical characterization and clinical trials to demonstrate comparable safety and efficacy to their reference biologics, addressing immunogenicity and pharmacokinetic profiles specifically. Generic drugs, being small molecules, require simpler bioequivalence studies without extensive clinical trials due to their identical chemical structures. Regulatory pathways demand more comprehensive clinical testing for biosimilars to ensure no clinically meaningful differences, whereas generics rely primarily on established bioequivalence parameters.
Market Approval and Patent Considerations
Biosimilars undergo a rigorous market approval process involving extensive comparability studies to demonstrate similarity to reference biologics, whereas generics require bioequivalence evidence with small-molecule drugs. Patent considerations for biosimilars are complex due to biologics' multifaceted structures and longer patent protection periods, often necessitating strategic litigation or licensing agreements. Regulatory pathways, such as the FDA's 351(k) biosimilar pathway and the Hatch-Waxman Act for generics, dictate distinct approval timelines and exclusivity periods that impact market entry and competition dynamics.
Cost Implications and Market Impact
Biosimilars typically incur higher development and manufacturing costs compared to generics due to complex biological structures and rigorous regulatory requirements, influencing pricing strategies and market access. Generics, with simplified chemical synthesis, offer lower production costs, leading to more aggressive price competition and broader market penetration. The introduction of biosimilars fosters cost savings and accessibility in biologic therapies but achieves slower market impact relative to generics, which rapidly capture market share in small-molecule drug categories.
Physician and Patient Perspectives
Physicians consider biosimilars and generics differently due to the complex nature of biologics compared to chemical drugs, requiring rigorous clinical evidence to ensure safety and efficacy for patient use. Patients often perceive biosimilars with caution, given the variability in manufacturing processes and potential immunogenicity concerns, while generics are generally accepted as identical alternatives to brand-name drugs. Effective education on regulatory standards and post-marketing surveillance data is crucial to building trust among both physicians and patients in choosing biosimilars as cost-effective therapeutic options.
Challenges in Adoption and Substitution
Biosimilars face significant challenges in adoption and substitution due to their complex manufacturing processes and the inherent variability compared to original biologics, which complicates regulatory approval and physician acceptance. Unlike generics, biosimilars cannot be considered identical copies, leading to cautious prescribing and limited automatic substitution at the pharmacy level. Payer policies and patient trust also impact the uptake, as concerns over immunogenicity and therapeutic equivalence persist within clinical practice.
Future Trends in Biosimilars and Generics
Future trends in biosimilars emphasize advanced molecular characterization and personalized medicine integration, driving enhanced efficacy and safety profiles. Generics will continue to dominate markets through accelerated approval processes and cost-effective manufacturing technologies. Convergence of digital health and AI analytics is expected to further optimize development pipelines and post-market surveillance for both biosimilars and generics.
biosimilar vs generic Infographic
 techiny.com
techiny.com