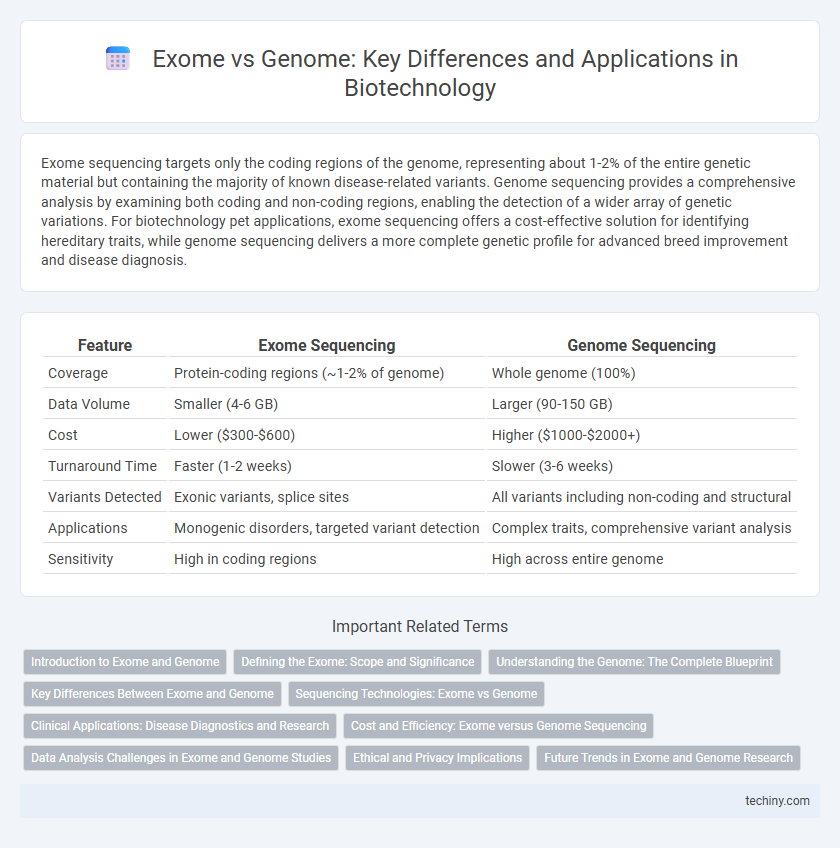
Exome sequencing targets only the coding regions of the genome, representing about 1-2% of the entire genetic material but containing the majority of known disease-related variants. Genome sequencing provides a comprehensive analysis by examining both coding and non-coding regions, enabling the detection of a wider array of genetic variations. For biotechnology pet applications, exome sequencing offers a cost-effective solution for identifying hereditary traits, while genome sequencing delivers a more complete genetic profile for advanced breed improvement and disease diagnosis.
Table of Comparison
| Feature | Exome Sequencing | Genome Sequencing |
|---|---|---|
| Coverage | Protein-coding regions (~1-2% of genome) | Whole genome (100%) |
| Data Volume | Smaller (4-6 GB) | Larger (90-150 GB) |
| Cost | Lower ($300-$600) | Higher ($1000-$2000+) |
| Turnaround Time | Faster (1-2 weeks) | Slower (3-6 weeks) |
| Variants Detected | Exonic variants, splice sites | All variants including non-coding and structural |
| Applications | Monogenic disorders, targeted variant detection | Complex traits, comprehensive variant analysis |
| Sensitivity | High in coding regions | High across entire genome |
Introduction to Exome and Genome
The genome encompasses the entire DNA sequence of an organism, including both coding and non-coding regions, totaling approximately 3 billion base pairs in humans. The exome represents the subset of the genome that consists of all exons, or protein-coding regions, which account for about 1-2% of the genome but harbor roughly 85% of disease-causing mutations. Exome sequencing is a targeted approach that enables efficient identification of genetic variants linked to disorders, while genome sequencing provides comprehensive coverage for a broader analysis of genetic information.
Defining the Exome: Scope and Significance
The exome represents the complete set of protein-coding regions within the genome, encompassing approximately 1-2% of the human genome but harboring around 85% of disease-causing mutations. Targeted sequencing of the exome provides a cost-effective approach to identify genetic variants linked to Mendelian disorders and complex diseases. Its focused scope allows researchers and clinicians to efficiently analyze functional genomic regions critical for understanding disease mechanisms and therapeutic targets.
Understanding the Genome: The Complete Blueprint
The genome encompasses the entire set of DNA, including all coding and non-coding regions, providing a comprehensive blueprint for an organism's biological functions. Exome sequencing targets only the protein-coding regions, representing approximately 1-2% of the genome but containing the majority of known disease-related variants. Complete genome analysis enables identification of regulatory elements, structural variants, and non-coding mutations critical for understanding complex genetic traits and personalized medicine.
Key Differences Between Exome and Genome
The exome comprises approximately 1-2% of the genome, focusing solely on protein-coding regions while the genome includes both coding and non-coding DNA sequences, totaling about 3 billion base pairs. Exome sequencing is more cost-effective and generates less data, making it ideal for identifying mutations linked to Mendelian disorders, whereas whole genome sequencing provides comprehensive analysis including regulatory elements and structural variants. The choice between exome and genome sequencing depends on clinical goals, such as targeted diagnosis versus broad discovery of genetic variations.
Sequencing Technologies: Exome vs Genome
Exome sequencing targets the protein-coding regions, constituting about 1-2% of the genome, using capture-based methods for cost-effective analysis of clinically relevant variants. Genome sequencing employs whole-genome sequencing (WGS) technologies, providing comprehensive coverage of coding and non-coding regions with higher data volume and greater variant detection capability. Advances in next-generation sequencing (NGS) platforms have improved accuracy and throughput for both exome and genome sequencing, enabling precise genetic diagnosis and research applications.
Clinical Applications: Disease Diagnostics and Research
Exome sequencing targets the protein-coding regions of the genome, enabling efficient identification of mutations linked to monogenic disorders, making it a cost-effective tool for clinical diagnostics. Genome sequencing offers comprehensive data, capturing both coding and non-coding variants, structural variations, and regulatory elements, enhancing the detection of complex genetic diseases and facilitating precision medicine. In research, whole-genome analysis provides a broader understanding of genetic contributions to disease mechanisms, while exome sequencing remains valuable for focused studies on coding mutations with high clinical relevance.
Cost and Efficiency: Exome versus Genome Sequencing
Exome sequencing targets the 1-2% of the genome that codes for proteins, making it a cost-effective option typically priced between $300 and $1,000, compared to whole genome sequencing which can range from $600 to $3,000. This focused approach yields higher coverage depth and faster analysis times, enhancing variant detection in coding regions. Whole genome sequencing, although more expensive and time-intensive, provides comprehensive data across coding and non-coding regions, enabling broader insights into genetic variation.
Data Analysis Challenges in Exome and Genome Studies
Exome and genome sequencing generate vast datasets requiring advanced bioinformatics tools for accurate variant calling and interpretation. Exome studies focus on coding regions, reducing data complexity yet facing challenges with uneven coverage and variant detection in non-coding regions. Genome sequencing provides comprehensive data but demands substantial computational resources to analyze regulatory elements and structural variants, complicating data integration and interpretation.
Ethical and Privacy Implications
Exome sequencing, targeting only the protein-coding regions of the genome, raises ethical concerns due to the selective disclosure of potentially significant genetic information that may impact privacy and informed consent processes. Whole genome sequencing provides a comprehensive genetic profile, increasing the risk of revealing incidental findings and sensitive data, which complicates data protection and patient autonomy. Strict regulatory frameworks and robust data encryption methods are essential to mitigate privacy breaches and ensure ethical handling of genetic information in both exome and genome analyses.
Future Trends in Exome and Genome Research
Advancements in CRISPR and next-generation sequencing are accelerating the shift from exome sequencing to comprehensive genome analysis, enabling unprecedented insights into genetic variations beyond coding regions. Increasing integration of artificial intelligence and machine learning algorithms is enhancing interpretation accuracy of both exomic and genomic data, facilitating personalized medicine and early disease prediction. Future research trends emphasize multi-omics approaches combining exome, genome, transcriptome, and epigenome data to unravel complex genetic traits and optimize therapeutic interventions.
Exome vs Genome Infographic
 techiny.com
techiny.com