Flow cytometry enables rapid analysis of physical and chemical characteristics of cells or particles, while Fluorescence-Activated Cell Sorting (FACS) extends this technology by isolating specific cell populations based on fluorescent labeling. FACS offers a powerful tool for purifying targeted cell subsets for downstream applications, which is essential in biotechnology research and pet diagnostics. Understanding the distinct roles of flow cytometry and FACS improves experimental precision and enhances the development of pet therapies.
Table of Comparison
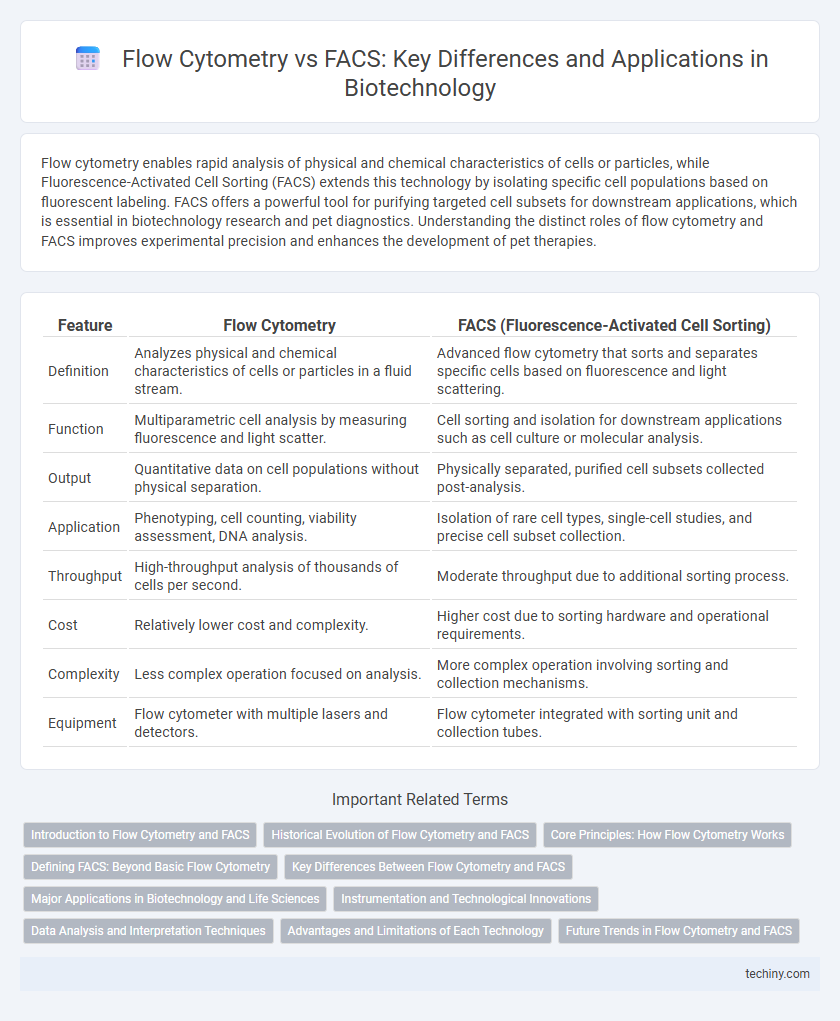
| Feature | Flow Cytometry | FACS (Fluorescence-Activated Cell Sorting) |
|---|---|---|
| Definition | Analyzes physical and chemical characteristics of cells or particles in a fluid stream. | Advanced flow cytometry that sorts and separates specific cells based on fluorescence and light scattering. |
| Function | Multiparametric cell analysis by measuring fluorescence and light scatter. | Cell sorting and isolation for downstream applications such as cell culture or molecular analysis. |
| Output | Quantitative data on cell populations without physical separation. | Physically separated, purified cell subsets collected post-analysis. |
| Application | Phenotyping, cell counting, viability assessment, DNA analysis. | Isolation of rare cell types, single-cell studies, and precise cell subset collection. |
| Throughput | High-throughput analysis of thousands of cells per second. | Moderate throughput due to additional sorting process. |
| Cost | Relatively lower cost and complexity. | Higher cost due to sorting hardware and operational requirements. |
| Complexity | Less complex operation focused on analysis. | More complex operation involving sorting and collection mechanisms. |
| Equipment | Flow cytometer with multiple lasers and detectors. | Flow cytometer integrated with sorting unit and collection tubes. |
Introduction to Flow Cytometry and FACS
Flow cytometry is a powerful technique that enables the rapid analysis of physical and chemical characteristics of cells or particles as they flow in a fluid stream through a laser beam. Fluorescence-activated cell sorting (FACS) builds on flow cytometry by adding the capability to sort and isolate specific cell populations based on fluorescent markers. These technologies are widely used in biotechnology for applications such as immunophenotyping, cell cycle analysis, and biomarker discovery.
Historical Evolution of Flow Cytometry and FACS
Flow cytometry originated in the late 1960s as a technique to rapidly analyze the physical and chemical properties of cells in suspension, revolutionizing cellular analysis with its ability to measure multiple parameters simultaneously. Fluorescence-activated cell sorting (FACS) emerged in the early 1970s as an advancement of flow cytometry, enabling not only the analysis but also the physical separation of cells based on fluorescent labeling. This evolution enhanced applications in immunology, cancer research, and stem cell biology by allowing precise isolation of specific cell populations.
Core Principles: How Flow Cytometry Works
Flow cytometry analyzes physical and chemical characteristics of cells or particles by suspending them in a fluid stream and passing them through a laser beam to detect light scattering and fluorescence signals. The core principle involves hydrodynamic focusing to align cells in a single file, enabling precise measurement of cell size, granularity, and fluorescence intensity from labeled antibodies or dyes. This technology allows rapid multiparametric analysis essential for immunophenotyping, cell sorting, and functional assays in biotechnology research.
Defining FACS: Beyond Basic Flow Cytometry
Fluorescence-Activated Cell Sorting (FACS) advances beyond traditional flow cytometry by enabling both the analysis and physical separation of cells based on fluorescent labeling. Unlike flow cytometry, which primarily provides quantitative data on cell populations, FACS employs electrostatic deflection to isolate specific cell subsets for downstream applications. This precise sorting capability makes FACS essential for applications like immunophenotyping, stem cell research, and single-cell genomics.
Key Differences Between Flow Cytometry and FACS
Flow Cytometry quantitatively measures physical and chemical characteristics of cells or particles in a fluid as they pass through a laser, providing data on size, granularity, and fluorescence intensity. Fluorescence-Activated Cell Sorting (FACS), a specialized type of flow cytometry, not only analyzes but also physically separates cells based on fluorescent labeling, enabling isolation of specific subpopulations for further study. Key differences include FACS's ability for cell sorting and recovery, whereas standard flow cytometry is limited to multiparametric analysis without cell isolation capabilities.
Major Applications in Biotechnology and Life Sciences
Flow cytometry enables rapid multiparametric analysis of thousands of cells per second, making it essential for immunophenotyping, cell cycle analysis, and biomarker detection in biotechnology and life sciences. Fluorescence-activated cell sorting (FACS), a specialized type of flow cytometry, allows precise sorting and isolation of specific cell populations based on fluorescent labeling, advancing stem cell research, cancer biology, and gene expression studies. Both techniques drive innovations in drug development and diagnostics by providing high-throughput, single-cell resolution data critical for understanding cellular heterogeneity and function.
Instrumentation and Technological Innovations
Flow Cytometry employs laser-based instruments to analyze the physical and chemical characteristics of cells or particles in a fluid stream, facilitating high-throughput multiparametric analysis. Fluorescence-Activated Cell Sorting (FACS), a specialized type of flow cytometry, integrates cell sorting capabilities using electrostatic deflection plates that direct charged droplets into collection tubes based on fluorescent markers. Recent technological innovations in Flow Cytometry and FACS include advancements in multi-laser platforms, enhanced sensitivity detectors, and microfluidic integration enabling higher precision, faster sorting speeds, and greater multiplexing capacity for complex cellular analyses.
Data Analysis and Interpretation Techniques
Flow Cytometry provides rapid multiparametric data acquisition allowing quantitative analysis of cell populations based on fluorescent markers, enabling detailed phenotypic profiling. Fluorescence-Activated Cell Sorting (FACS) extends this capability by physically isolating specific cell subsets for downstream functional assays, thus enhancing data interpretation with sorted population-specific insights. Advanced computational algorithms, including machine learning and high-dimensional data visualization tools like t-SNE and UMAP, are increasingly integrated to improve accuracy in identifying rare cell types and understanding complex biological heterogeneity.
Advantages and Limitations of Each Technology
Flow cytometry offers rapid multiparametric analysis of thousands of cells per second, enabling high-throughput quantification of cell size, granularity, and fluorescent markers; however, it lacks the ability to physically separate and collect specific cell populations. Fluorescence-activated cell sorting (FACS) extends flow cytometry by providing precise sorting and isolation of targeted cells based on fluorescence characteristics, crucial for downstream applications such as cell culture or genetic analysis, though this method involves greater complexity, cost, and requires meticulous calibration. Both technologies are indispensable in immunology and cancer research, with flow cytometry favored for analytical purposes and FACS for functional studies requiring pure cell subsets.
Future Trends in Flow Cytometry and FACS
Flow cytometry and FACS are rapidly evolving with advancements in multiparametric analysis and real-time cell sorting enhancing precision in cellular phenotyping. Integration of artificial intelligence and machine learning algorithms is poised to revolutionize data interpretation, enabling deeper insights into heterogeneous cell populations. Emerging trends emphasize miniaturization and microfluidic platforms, promising more accessible and high-throughput applications in clinical diagnostics and personalized medicine.
Flow Cytometry vs FACS Infographic
 techiny.com
techiny.com