Gene knockout permanently disables a specific gene, completely eliminating its function to study the effects on an organism or develop new treatments. In contrast, gene knockdown reduces gene expression temporarily or partially, allowing researchers to observe changes without fully abolishing gene activity. Both techniques are crucial for advancing biotechnology in pets, enabling targeted genetic studies and potential therapies for hereditary conditions.
Table of Comparison
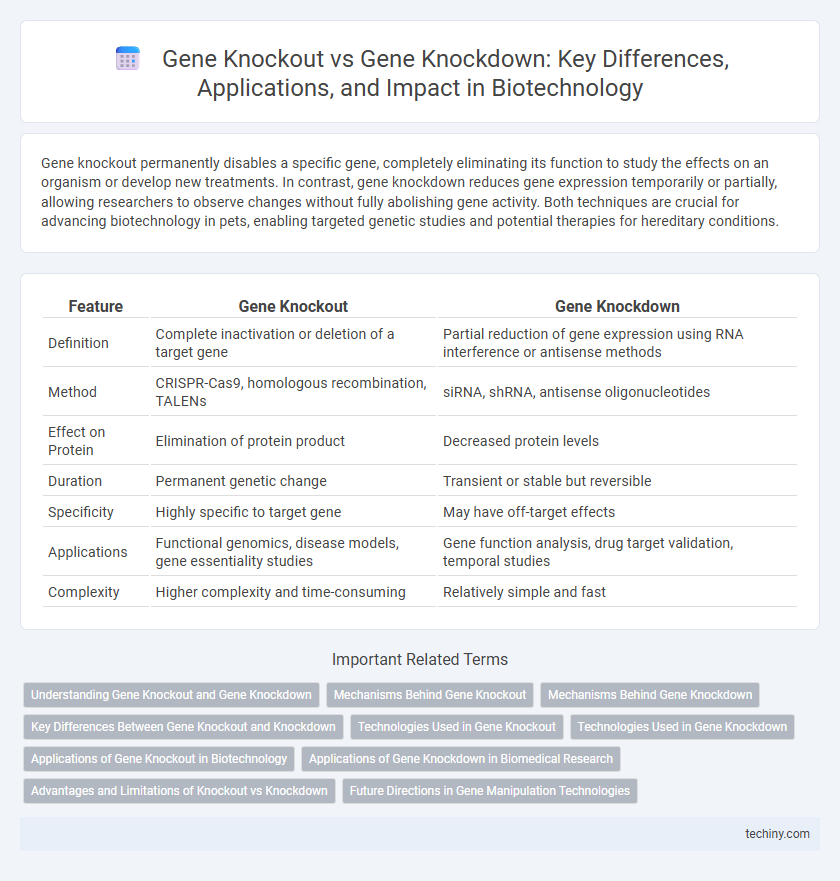
| Feature | Gene Knockout | Gene Knockdown |
|---|---|---|
| Definition | Complete inactivation or deletion of a target gene | Partial reduction of gene expression using RNA interference or antisense methods |
| Method | CRISPR-Cas9, homologous recombination, TALENs | siRNA, shRNA, antisense oligonucleotides |
| Effect on Protein | Elimination of protein product | Decreased protein levels |
| Duration | Permanent genetic change | Transient or stable but reversible |
| Specificity | Highly specific to target gene | May have off-target effects |
| Applications | Functional genomics, disease models, gene essentiality studies | Gene function analysis, drug target validation, temporal studies |
| Complexity | Higher complexity and time-consuming | Relatively simple and fast |
Understanding Gene Knockout and Gene Knockdown
Gene knockout involves the complete and permanent inactivation of a specific gene, often achieved using CRISPR-Cas9 or homologous recombination techniques, resulting in the total loss of gene function. Gene knockdown refers to the partial and reversible reduction of gene expression, commonly utilizing RNA interference (RNAi) or antisense oligonucleotides to suppress mRNA levels without altering the genomic DNA. Understanding the distinctions between gene knockout and gene knockdown is essential for designing experiments that require precise control over gene activity and functional analysis in biotechnology research.
Mechanisms Behind Gene Knockout
Gene knockout involves permanently disabling a specific gene by introducing targeted mutations or deletions at the DNA level, often using CRISPR-Cas9 or homologous recombination techniques. This process disrupts the gene's coding sequence, preventing the production of its functional protein entirely. The complete loss of gene function allows researchers to study gene roles by analyzing phenotypic changes in knockout organisms or cells.
Mechanisms Behind Gene Knockdown
Gene knockdown reduces gene expression by interfering with mRNA stability or translation, primarily through mechanisms such as RNA interference (RNAi) involving small interfering RNA (siRNA) or short hairpin RNA (shRNA). These RNA molecules guide the RNA-induced silencing complex (RISC) to degrade target mRNA or inhibit its translation without altering the DNA sequence. This reversible and partial suppression contrasts with gene knockout, which permanently disrupts the gene at the DNA level using techniques like CRISPR-Cas9 or homologous recombination.
Key Differences Between Gene Knockout and Knockdown
Gene knockout involves the complete inactivation or deletion of a specific gene, resulting in a permanent loss of gene function, whereas gene knockdown reduces gene expression temporarily or partially without altering the DNA sequence. Knockout techniques often utilize CRISPR-Cas9 or homologous recombination to achieve a full gene disruption, while knockdown typically employs RNA interference (RNAi) or antisense oligonucleotides for transient suppression. The key differences lie in knockout's permanent effect and complete gene silencing versus knockdown's reversible and partial gene expression reduction, impacting experimental design and therapeutic strategies in biotechnology.
Technologies Used in Gene Knockout
Gene knockout technology predominantly employs CRISPR-Cas9, zinc finger nucleases (ZFNs), and transcription activator-like effector nucleases (TALENs) to achieve precise genomic deletions by inducing double-strand breaks in DNA. These tools introduce targeted mutations that permanently inactivate specific genes, allowing researchers to study gene function through complete loss-of-function models. CRISPR-Cas9, known for its efficiency and versatility, has become the most widely adopted method due to its RNA-guided DNA cleavage mechanism and ease of design.
Technologies Used in Gene Knockdown
Gene knockdown primarily employs RNA interference (RNAi) technologies such as small interfering RNA (siRNA) and short hairpin RNA (shRNA) to transiently reduce gene expression by degrading target mRNA. CRISPR interference (CRISPRi) utilizes catalytically dead Cas9 (dCas9) fused with repressor domains to inhibit transcription without altering the DNA sequence. These technologies enable precise, reversible gene regulation, contrasting with gene knockout methods that involve permanent DNA modification through CRISPR-Cas9 or homologous recombination.
Applications of Gene Knockout in Biotechnology
Gene knockout techniques enable precise removal or disruption of specific genes, offering powerful applications in biotechnology such as creating genetically modified organisms (GMOs) with desirable traits, studying gene functions, and developing disease models for pharmaceutical research. By permanently altering the genome, gene knockout facilitates the production of crops with enhanced resistance to pests, improved nutritional content, and increased yield. This approach also accelerates drug discovery by enabling the identification of target genes and pathways involved in diseases through in vivo functional genomic studies.
Applications of Gene Knockdown in Biomedical Research
Gene knockdown techniques, such as RNA interference (RNAi) and antisense oligonucleotides, enable selective reduction of gene expression, facilitating the study of gene function in disease models. These methods are widely applied in cancer research to inhibit oncogene expression and assess tumor growth dynamics. Gene knockdown also aids in drug target validation and the investigation of complex genetic pathways involved in neurological disorders and infectious diseases.
Advantages and Limitations of Knockout vs Knockdown
Gene knockout offers complete and permanent gene inactivation, enabling clear analysis of gene function and phenotypic consequences, but it can lead to compensatory mechanisms and embryonic lethality in essential genes. Gene knockdown provides transient and partial suppression of gene expression through RNA interference or antisense oligonucleotides, allowing reversible studies and dosage effects but often results in incomplete knockdown and off-target effects. Knockout models are preferable for studying gene essentiality and long-term effects, whereas knockdown approaches are advantageous for temporal control and reducing potential adaptive responses.
Future Directions in Gene Manipulation Technologies
Emerging gene manipulation technologies are advancing beyond traditional gene knockout and gene knockdown methods by integrating CRISPR-based base editing and prime editing, enabling precise and efficient modifications without inducing double-strand breaks. Future directions emphasize developing programmable epigenome editing tools that modulate gene expression temporarily, offering reversible therapeutic applications with reduced off-target effects. These innovations promise transformative impacts in functional genomics, personalized medicine, and synthetic biology by enhancing specificity, scalability, and control over gene regulation.
Gene knockout vs Gene knockdown Infographic
 techiny.com
techiny.com