RNA interference (RNAi) and CRISPR are powerful biotechnological tools used for gene editing and regulation in pets. RNAi selectively silences specific gene expression by degrading targeted mRNA molecules, providing a reversible and precise method for controlling protein production. CRISPR offers a more permanent solution by directly modifying the genome, allowing for targeted gene knockout or insertion with high accuracy and efficiency, revolutionizing pet disease research and genetic improvement.
Table of Comparison
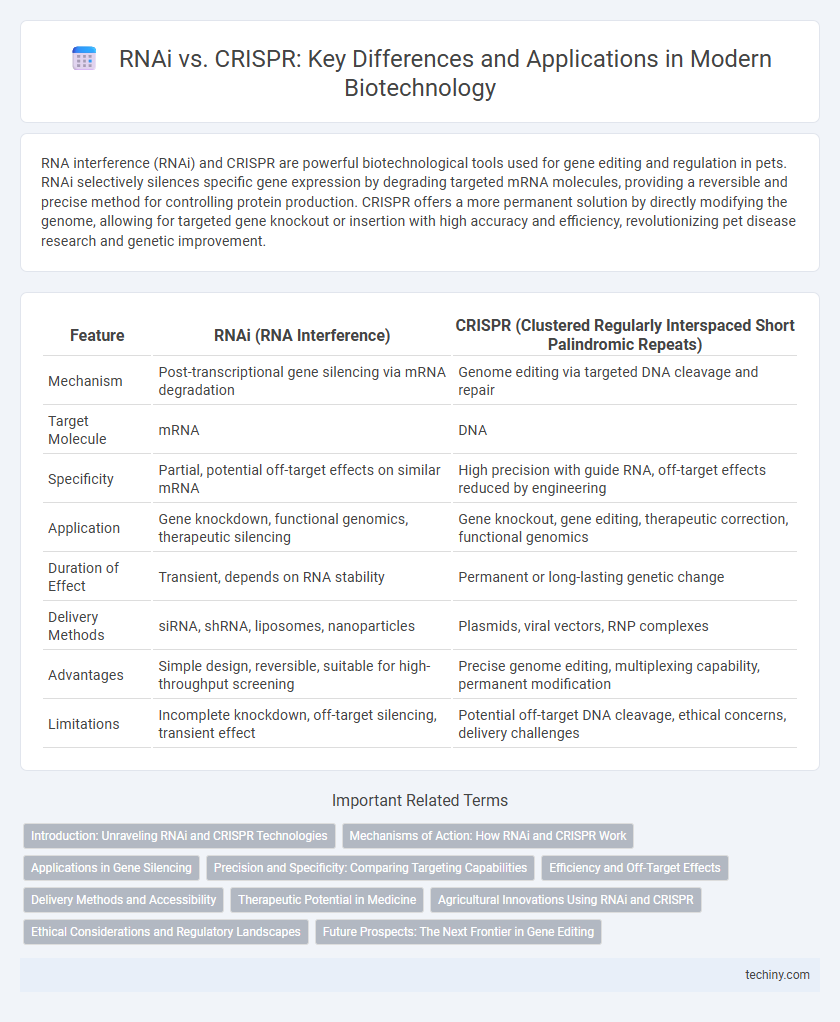
| Feature | RNAi (RNA Interference) | CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) |
|---|---|---|
| Mechanism | Post-transcriptional gene silencing via mRNA degradation | Genome editing via targeted DNA cleavage and repair |
| Target Molecule | mRNA | DNA |
| Specificity | Partial, potential off-target effects on similar mRNA | High precision with guide RNA, off-target effects reduced by engineering |
| Application | Gene knockdown, functional genomics, therapeutic silencing | Gene knockout, gene editing, therapeutic correction, functional genomics |
| Duration of Effect | Transient, depends on RNA stability | Permanent or long-lasting genetic change |
| Delivery Methods | siRNA, shRNA, liposomes, nanoparticles | Plasmids, viral vectors, RNP complexes |
| Advantages | Simple design, reversible, suitable for high-throughput screening | Precise genome editing, multiplexing capability, permanent modification |
| Limitations | Incomplete knockdown, off-target silencing, transient effect | Potential off-target DNA cleavage, ethical concerns, delivery challenges |
Introduction: Unraveling RNAi and CRISPR Technologies
RNA interference (RNAi) and CRISPR-Cas systems both serve as powerful tools for gene regulation and editing, leveraging distinct molecular mechanisms to manipulate genetic material. RNAi employs small interfering RNAs (siRNAs) to silence gene expression post-transcriptionally by degrading target mRNA, while CRISPR utilizes guide RNAs to direct Cas nucleases for precise DNA cleavage and genome modification. Understanding the biochemical pathways and applications of RNAi and CRISPR highlights their transformative roles in biotechnology, gene therapy, and functional genomics.
Mechanisms of Action: How RNAi and CRISPR Work
RNA interference (RNAi) operates by degrading specific messenger RNA (mRNA) molecules, thereby preventing the translation of target genes into proteins through the introduction of small interfering RNA (siRNA) or microRNA (miRNA) sequences. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) utilizes a guide RNA to direct the Cas9 endonuclease to exact DNA sequences for targeted double-strand breaks, enabling gene editing through non-homologous end joining or homology-directed repair. RNAi modulates gene expression post-transcriptionally without altering the genome, while CRISPR enables permanent genomic modifications at the DNA level.
Applications in Gene Silencing
RNA interference (RNAi) enables targeted gene silencing by degrading specific mRNA molecules, effectively reducing gene expression in therapeutic and agricultural applications. CRISPR technology employs a guide RNA and Cas proteins to create precise DNA double-strand breaks, facilitating permanent gene knockout or transcriptional repression in gene editing. While RNAi offers transient and reversible gene silencing useful for functional genomics, CRISPR provides durable and highly specific genome modifications essential for treating genetic disorders and engineering crops.
Precision and Specificity: Comparing Targeting Capabilities
RNAi leverages small interfering RNA molecules to degrade specific mRNA sequences, achieving post-transcriptional gene silencing with moderate specificity but potential off-target effects. CRISPR-Cas9 employs guide RNA to direct Cas9 nuclease precisely to DNA sequences, enabling permanent gene edits with higher precision and reduced off-target activity. The superior targeting accuracy of CRISPR enhances its utility for therapeutic gene editing and functional genomics compared to RNAi's transient and less specific silencing mechanisms.
Efficiency and Off-Target Effects
RNA interference (RNAi) achieves gene silencing by degrading target mRNA, often resulting in partial knockdown and variable efficiency across different cell types. CRISPR-Cas9 offers higher precision and efficiency by directly editing genomic DNA, enabling permanent gene modifications with typically lower off-target effects when optimized guide RNAs are used. Comparative studies reveal that CRISPR systems generally demonstrate more robust on-target activity and reduced unintended gene alterations compared to RNAi, making CRISPR a preferred tool for therapeutic gene editing.
Delivery Methods and Accessibility
RNA interference (RNAi) delivery relies heavily on lipid nanoparticles and viral vectors for efficient intracellular transport, demonstrating relatively simpler synthesis and transient gene silencing effects, making it accessible for therapeutic applications targeting specific mRNA. CRISPR systems, particularly CRISPR-Cas9, utilize electroporation, viral vectors, and ribonucleoprotein complexes to introduce precise genome edits, but challenges in delivery efficiency, off-target effects, and immunogenicity limit widespread clinical accessibility. Innovations in non-viral delivery and nanoparticle engineering are narrowing the gap, yet RNAi remains more accessible currently due to established delivery platforms and regulatory familiarity.
Therapeutic Potential in Medicine
RNA interference (RNAi) and CRISPR-Cas9 both offer groundbreaking therapeutic potential by enabling precise gene regulation and editing, respectively, to target genetic diseases. RNAi technology silences disease-causing genes post-transcriptionally, proving effective in treating viral infections and cancers by reducing harmful protein expression. CRISPR's ability to directly edit DNA sequences allows for permanent correction of genetic mutations, showing promise in inherited disorders like cystic fibrosis and sickle cell anemia.
Agricultural Innovations Using RNAi and CRISPR
RNA interference (RNAi) enables precise gene silencing to enhance crop resistance against pests and diseases without altering the genome, offering a non-transgenic approach in agriculture. CRISPR-Cas9 technology allows targeted genome editing to create crops with improved yield, stress tolerance, and nutritional profiles by introducing or knocking out specific genes. Combining RNAi and CRISPR accelerates the development of sustainable agricultural innovations by integrating gene regulation and genome editing for crop improvement.
Ethical Considerations and Regulatory Landscapes
RNAi and CRISPR technologies present distinct ethical considerations, with CRISPR's potential for permanent genome editing raising significant concerns about unintended mutations and gene drives, while RNAi's transient gene silencing is generally viewed as lower risk. Regulatory landscapes vary globally, with CRISPR facing stricter oversight due to its gene-editing capabilities, exemplified by the U.S. FDA's cautious approach and the European Union's stringent GMO regulations. Ethical debates focus on human germline modifications and ecological impacts, prompting calls for international consensus and robust frameworks to ensure responsible use of both RNAi and CRISPR in biotechnology.
Future Prospects: The Next Frontier in Gene Editing
RNAi and CRISPR represent two revolutionary gene-editing technologies with distinct mechanisms and applications, where CRISPR offers precise DNA-level modifications while RNAi regulates gene expression at the mRNA level. Future prospects in biotechnology emphasize CRISPR's potential for treating genetic disorders, enabling multiplexed gene editing, and advancing synthetic biology through programmable nucleases. RNAi continues to hold promise in therapeutic gene silencing and functional genomics, but CRISPR's versatility and efficiency mark the next frontier in gene editing innovation.
RNAi vs CRISPR Infographic
 techiny.com
techiny.com