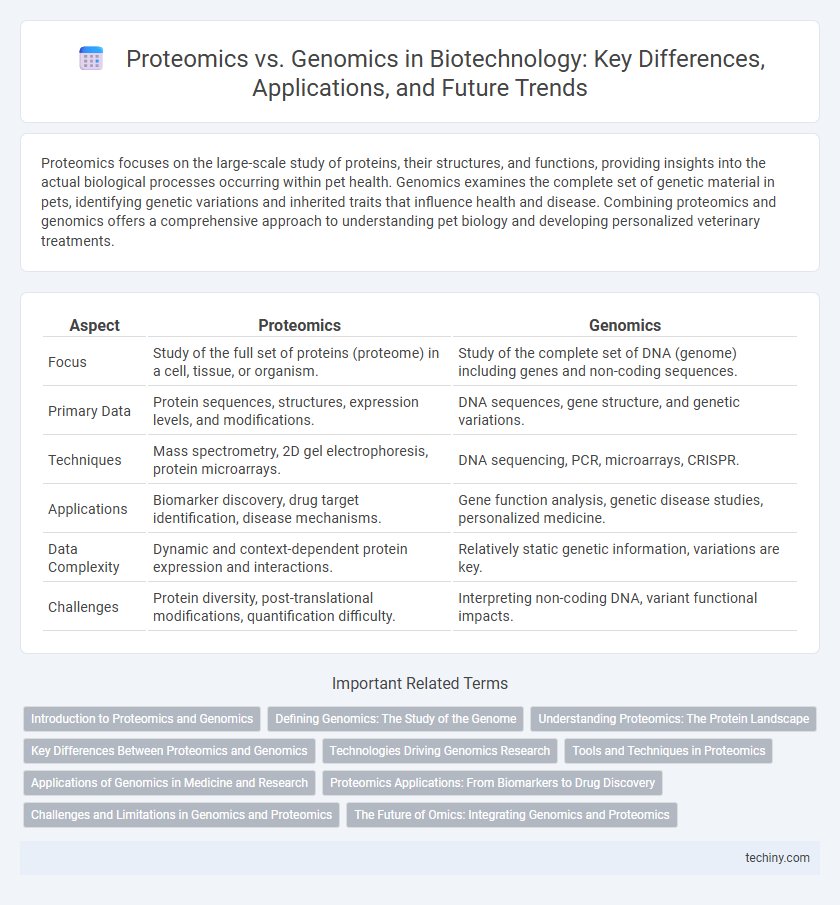
Proteomics focuses on the large-scale study of proteins, their structures, and functions, providing insights into the actual biological processes occurring within pet health. Genomics examines the complete set of genetic material in pets, identifying genetic variations and inherited traits that influence health and disease. Combining proteomics and genomics offers a comprehensive approach to understanding pet biology and developing personalized veterinary treatments.
Table of Comparison
| Aspect | Proteomics | Genomics |
|---|---|---|
| Focus | Study of the full set of proteins (proteome) in a cell, tissue, or organism. | Study of the complete set of DNA (genome) including genes and non-coding sequences. |
| Primary Data | Protein sequences, structures, expression levels, and modifications. | DNA sequences, gene structure, and genetic variations. |
| Techniques | Mass spectrometry, 2D gel electrophoresis, protein microarrays. | DNA sequencing, PCR, microarrays, CRISPR. |
| Applications | Biomarker discovery, drug target identification, disease mechanisms. | Gene function analysis, genetic disease studies, personalized medicine. |
| Data Complexity | Dynamic and context-dependent protein expression and interactions. | Relatively static genetic information, variations are key. |
| Challenges | Protein diversity, post-translational modifications, quantification difficulty. | Interpreting non-coding DNA, variant functional impacts. |
Introduction to Proteomics and Genomics
Proteomics involves the large-scale study of proteins, their structures, functions, and interactions within a biological system, providing critical insights into cellular processes and disease mechanisms. Genomics focuses on the comprehensive analysis of an organism's entire genome, including DNA sequencing, gene mapping, and functional genomics to understand genetic contributions to phenotype and health. Both fields employ high-throughput technologies like mass spectrometry in proteomics and next-generation sequencing in genomics, driving advances in personalized medicine and biomarker discovery.
Defining Genomics: The Study of the Genome
Genomics involves the comprehensive analysis of an organism's entire genome, encompassing sequencing, mapping, and functional characterization of genes. It investigates gene structure, expression, regulation, and interactions to understand hereditary traits and genetic variation. This holistic approach contrasts with proteomics, which focuses on the study of the complete set of proteins expressed by the genome under specific conditions.
Understanding Proteomics: The Protein Landscape
Proteomics explores the entire protein landscape, revealing the dynamic structures, functions, and interactions of proteins in biological systems. Unlike genomics, which maps the static DNA sequences, proteomics captures the real-time expression and modifications of proteins, crucial for understanding cellular mechanisms and disease pathways. Advanced mass spectrometry and bioinformatics tools enable comprehensive protein identification and quantification, facilitating biomarker discovery and personalized medicine.
Key Differences Between Proteomics and Genomics
Proteomics involves the large-scale study of proteins, including their structure, function, and interactions, while genomics focuses on analyzing the complete set of DNA within an organism to understand gene sequences and functions. Proteomics provides insights into protein expression patterns and post-translational modifications, offering dynamic information beyond static genomic data. Genomics serves as a foundational blueprint for biological processes, whereas proteomics reveals the actual biological activity and molecular mechanisms driving cellular function.
Technologies Driving Genomics Research
Next-generation sequencing (NGS) and CRISPR-Cas9 gene editing are pivotal technologies driving genomics research, enabling rapid, high-throughput analysis of DNA sequences and precise genome modifications. Bioinformatics tools and machine learning algorithms enhance data interpretation, facilitating discoveries in gene function, variation, and disease associations. These advancements accelerate personalized medicine, evolutionary studies, and functional genomics by providing comprehensive insights into the complexities of the genome.
Tools and Techniques in Proteomics
Proteomics employs advanced tools such as mass spectrometry, two-dimensional gel electrophoresis, and protein microarrays to analyze protein structure, function, and interactions at a large scale. Techniques like tandem mass spectrometry (MS/MS) enable precise identification and quantification of proteins in complex biological samples, while isotope labeling methods enhance accuracy in comparative proteomic studies. Bioinformatics software plays a crucial role in interpreting proteomic data, facilitating protein database searches and pathway analysis to understand cellular processes and disease mechanisms.
Applications of Genomics in Medicine and Research
Genomics plays a crucial role in personalized medicine by enabling the identification of genetic mutations responsible for diseases, facilitating targeted therapies and predictive diagnostics. In research, genomic sequencing accelerates the discovery of novel biomarkers and therapeutic targets, enhancing drug development and disease prevention strategies. The integration of genomics in clinical settings improves patient outcomes through precision medicine, genetic risk assessment, and tailored treatment plans.
Proteomics Applications: From Biomarkers to Drug Discovery
Proteomics enables the large-scale study of proteins, offering critical insights beyond genomics by identifying disease biomarkers and understanding protein functions in cellular processes. Advanced proteomic technologies such as mass spectrometry and protein microarrays accelerate drug discovery by revealing novel therapeutic targets and mechanisms of drug action. These applications drive personalized medicine, improving diagnostic accuracy and enabling the development of targeted therapies in biotechnology.
Challenges and Limitations in Genomics and Proteomics
Genomics faces challenges including the complexity of interpreting vast amounts of sequence data, difficulties in identifying functional elements within non-coding regions, and limitations in detecting epigenetic modifications. Proteomics struggles with the dynamic range of protein expression, post-translational modifications, and the incomplete coverage of the proteome due to technical constraints in mass spectrometry-based detection. Both fields encounter issues related to data integration, reproducibility, and the high cost of advanced experimental technologies.
The Future of Omics: Integrating Genomics and Proteomics
Integrating genomics and proteomics is revolutionizing precision medicine by enabling comprehensive analysis of DNA sequences alongside protein expressions and interactions, thus providing a holistic understanding of cellular functions. Advances in multi-omics technologies, such as mass spectrometry combined with next-generation sequencing, facilitate simultaneous profiling of genes and proteins to identify novel biomarkers and therapeutic targets. This synergistic approach accelerates drug discovery and personalized treatment strategies by bridging the gap between genotype and phenotype in complex diseases.
Proteomics vs Genomics Infographic
 techiny.com
techiny.com