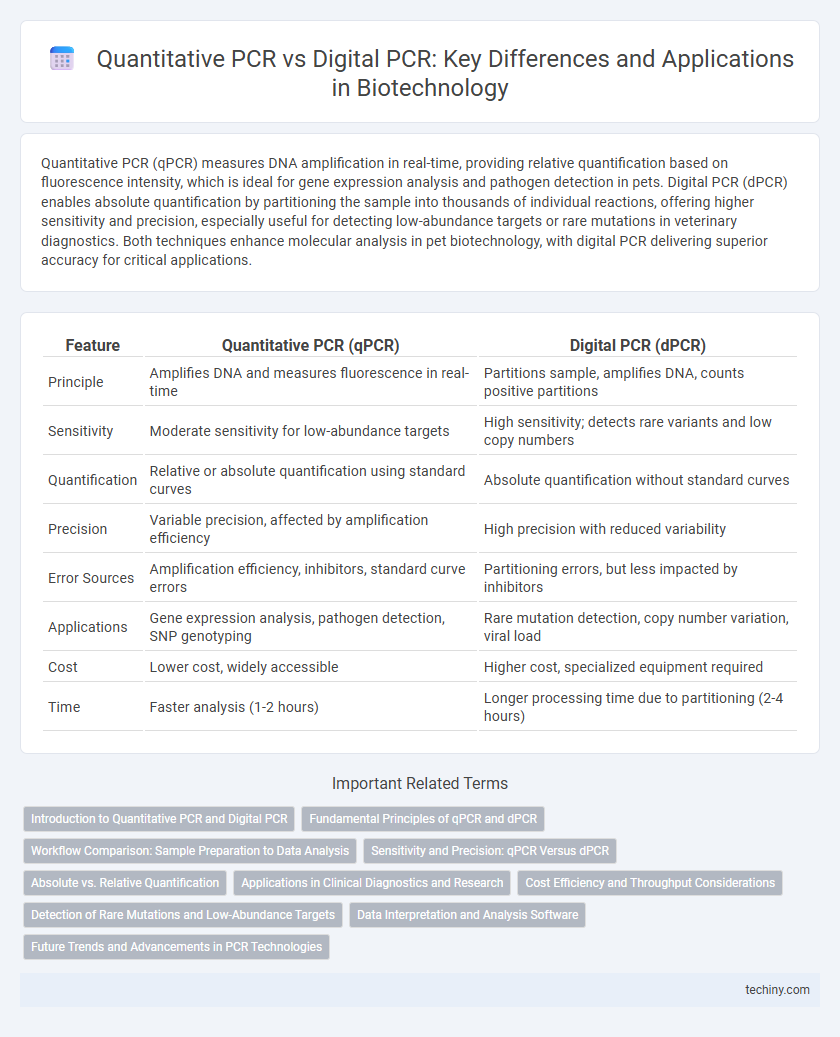
Quantitative PCR (qPCR) measures DNA amplification in real-time, providing relative quantification based on fluorescence intensity, which is ideal for gene expression analysis and pathogen detection in pets. Digital PCR (dPCR) enables absolute quantification by partitioning the sample into thousands of individual reactions, offering higher sensitivity and precision, especially useful for detecting low-abundance targets or rare mutations in veterinary diagnostics. Both techniques enhance molecular analysis in pet biotechnology, with digital PCR delivering superior accuracy for critical applications.
Table of Comparison
| Feature | Quantitative PCR (qPCR) | Digital PCR (dPCR) |
|---|---|---|
| Principle | Amplifies DNA and measures fluorescence in real-time | Partitions sample, amplifies DNA, counts positive partitions |
| Sensitivity | Moderate sensitivity for low-abundance targets | High sensitivity; detects rare variants and low copy numbers |
| Quantification | Relative or absolute quantification using standard curves | Absolute quantification without standard curves |
| Precision | Variable precision, affected by amplification efficiency | High precision with reduced variability |
| Error Sources | Amplification efficiency, inhibitors, standard curve errors | Partitioning errors, but less impacted by inhibitors |
| Applications | Gene expression analysis, pathogen detection, SNP genotyping | Rare mutation detection, copy number variation, viral load |
| Cost | Lower cost, widely accessible | Higher cost, specialized equipment required |
| Time | Faster analysis (1-2 hours) | Longer processing time due to partitioning (2-4 hours) |
Introduction to Quantitative PCR and Digital PCR
Quantitative PCR (qPCR) enables real-time monitoring of DNA amplification using fluorescent dyes or probes to quantify nucleic acids with high sensitivity and specificity. Digital PCR (dPCR) partitions the sample into thousands of individual reactions, allowing absolute quantification of target DNA molecules without the need for standard curves. Both techniques are essential in biotechnology for diagnostics, gene expression analysis, and precise mutation detection.
Fundamental Principles of qPCR and dPCR
Quantitative PCR (qPCR) measures DNA amplification in real-time by detecting fluorescence signals proportional to the amount of amplified target, enabling relative quantification based on threshold cycle values. Digital PCR (dPCR) partitions the sample into thousands of individual reactions, allowing absolute quantification of nucleic acids by counting positive partitions without reliance on standard curves. The fundamental principle of qPCR centers on exponential amplification monitored continuously, whereas dPCR focuses on end-point analysis of discrete reaction volumes for precise target molecule enumeration.
Workflow Comparison: Sample Preparation to Data Analysis
Quantitative PCR (qPCR) involves real-time amplification with fluorescence monitoring, requiring careful calibration curves for quantification, while digital PCR (dPCR) partitions samples into thousands of micro-reactions for absolute quantification without standard curves. Sample preparation for both methods is similar, involving nucleic acid extraction and purification; however, dPCR necessitates additional steps for sample partitioning using droplet or chip-based systems. Data analysis in qPCR relies on cycle threshold (Ct) values and relative quantification, whereas dPCR provides direct counts of target molecules, enabling higher precision and sensitivity, especially in detecting rare mutations or low-abundance targets.
Sensitivity and Precision: qPCR Versus dPCR
Digital PCR (dPCR) exhibits superior sensitivity and precision compared to quantitative PCR (qPCR) by partitioning the sample into thousands of micro-reactions, enabling absolute quantification of nucleic acids without reliance on standard curves. This partitioning reduces amplification bias and enhances the detection of low-abundance targets, making dPCR ideal for rare allele detection and minimal residual disease monitoring. In contrast, qPCR's relative quantification approach is more susceptible to variability and less effective in discriminating small fold changes in gene expression.
Absolute vs. Relative Quantification
Quantitative PCR (qPCR) primarily provides relative quantification by measuring the amplification of target DNA in real-time against a reference gene or control sample, allowing comparison of gene expression levels. Digital PCR (dPCR) enables absolute quantification by partitioning the sample into numerous individual reactions, directly counting the number of target DNA molecules without the need for standard curves. This absolute quantification with dPCR offers higher precision and sensitivity, particularly useful for detecting low-abundance targets or rare genetic variants in biotechnology applications.
Applications in Clinical Diagnostics and Research
Quantitative PCR (qPCR) enables precise quantification of nucleic acids in clinical diagnostics, facilitating disease detection, gene expression analysis, and pathogen identification with high sensitivity and specificity. Digital PCR (dPCR) offers absolute quantification without the need for standard curves, improving mutation detection, rare allele quantification, and copy number variation analysis in research and clinical settings. Both techniques are essential for personalized medicine, enabling accurate biomarker quantification and enhancing diagnostic accuracy in oncology, infectious diseases, and genetic disorders.
Cost Efficiency and Throughput Considerations
Quantitative PCR (qPCR) offers cost efficiency suitable for high-throughput gene expression analysis with moderate sensitivity, making it ideal for routine laboratory workflows. Digital PCR (dPCR) delivers absolute quantification with enhanced sensitivity and precision but incurs higher operational costs and lower sample throughput due to complex partitioning and instrumentation requirements. Selecting between qPCR and dPCR depends on balancing the need for cost-effective large-scale screening against the demand for highly accurate and sensitive target quantification in specialized applications.
Detection of Rare Mutations and Low-Abundance Targets
Digital PCR outperforms quantitative PCR in detecting rare mutations and low-abundance targets by partitioning samples into thousands of individual reactions, enabling precise absolute quantification without reliance on standard curves. This enhanced sensitivity allows for the identification of variants present at allele frequencies below 0.1%, critical for early cancer detection and minimal residual disease monitoring. Quantitative PCR's relative quantification and susceptibility to amplification efficiency variations limit its effectiveness in scenarios demanding ultra-sensitive mutation detection.
Data Interpretation and Analysis Software
Digital PCR offers enhanced precision in data interpretation through absolute quantification without reliance on standard curves, contrasting with quantitative PCR which requires relative quantification and is more susceptible to amplification efficiency variations. Advanced analysis software for digital PCR provides automated threshold setting, partition classification, and multiplexing capabilities, facilitating accurate detection of low-abundance targets and rare mutations. In contrast, qPCR software primarily focuses on cycle threshold (Ct) determination and melting curve analysis to estimate gene expression levels.
Future Trends and Advancements in PCR Technologies
Digital PCR (dPCR) is advancing rapidly with enhanced sensitivity and absolute quantification capabilities, positioning it as the future standard for precise genetic analysis. Innovations such as microfluidic integration and multiplexing expansion are driving higher throughput and cost-effectiveness, surpassing traditional quantitative PCR (qPCR) limitations. Emerging trends focus on automation and real-time data analytics to enable more accurate, reproducible, and scalable PCR applications in clinical diagnostics and personalized medicine.
quantitative PCR vs digital PCR Infographic
 techiny.com
techiny.com