Somatic Cell Nuclear Transfer (SCNT) involves transferring the nucleus of a somatic cell into an enucleated egg to produce a clone, whereas Embryonic Stem Cell Transfer (ESCT) introduces pluripotent stem cells into developing embryos to enhance genetic traits. SCNT offers precise genetic replication ideal for preserving valuable pet genetics, while ESCT enables integration of desirable traits without complete cloning. Both techniques revolutionize pet biotechnology by improving genetic diversity and disease resistance.
Table of Comparison
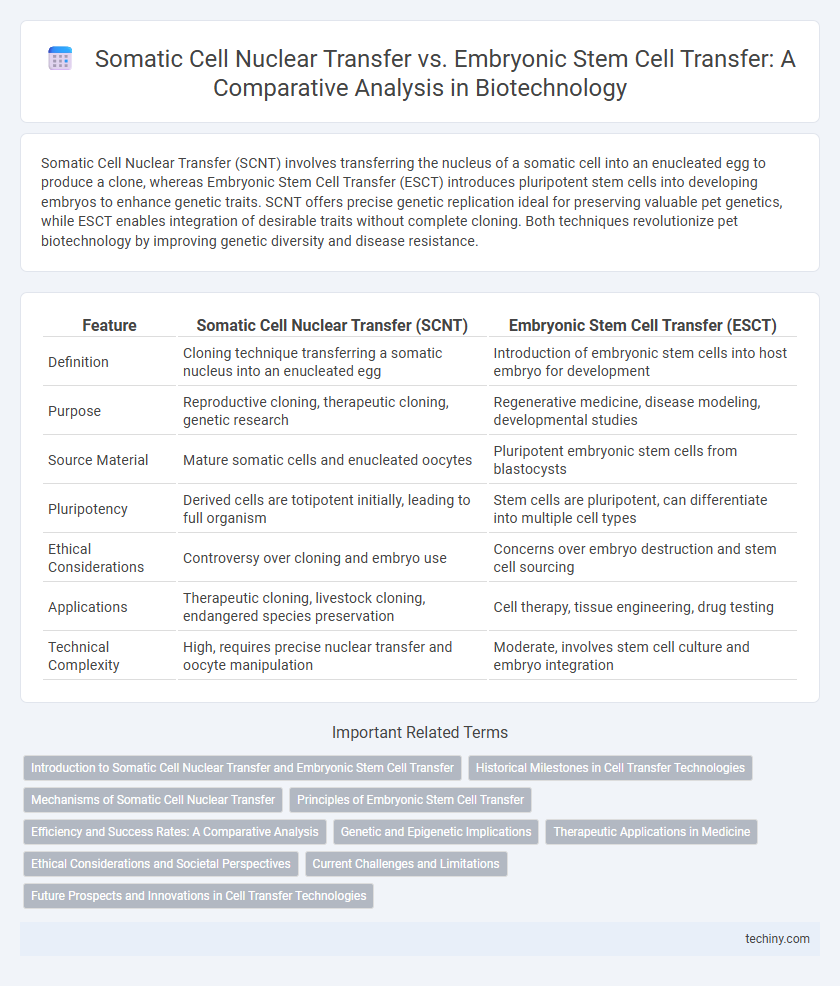
| Feature | Somatic Cell Nuclear Transfer (SCNT) | Embryonic Stem Cell Transfer (ESCT) |
|---|---|---|
| Definition | Cloning technique transferring a somatic nucleus into an enucleated egg | Introduction of embryonic stem cells into host embryo for development |
| Purpose | Reproductive cloning, therapeutic cloning, genetic research | Regenerative medicine, disease modeling, developmental studies |
| Source Material | Mature somatic cells and enucleated oocytes | Pluripotent embryonic stem cells from blastocysts |
| Pluripotency | Derived cells are totipotent initially, leading to full organism | Stem cells are pluripotent, can differentiate into multiple cell types |
| Ethical Considerations | Controversy over cloning and embryo use | Concerns over embryo destruction and stem cell sourcing |
| Applications | Therapeutic cloning, livestock cloning, endangered species preservation | Cell therapy, tissue engineering, drug testing |
| Technical Complexity | High, requires precise nuclear transfer and oocyte manipulation | Moderate, involves stem cell culture and embryo integration |
Introduction to Somatic Cell Nuclear Transfer and Embryonic Stem Cell Transfer
Somatic Cell Nuclear Transfer (SCNT) involves replacing the nucleus of an egg cell with the nucleus from a somatic cell, enabling the creation of a genetically identical organism and providing a powerful tool for cloning and therapeutic applications. Embryonic Stem Cell Transfer (ESCT) focuses on transferring pluripotent embryonic stem cells into host embryos to study development or regenerate tissues, leveraging their unique ability to differentiate into various cell types. Both techniques are central to regenerative medicine and developmental biology, offering distinct approaches to cellular reprogramming and tissue engineering.
Historical Milestones in Cell Transfer Technologies
Somatic Cell Nuclear Transfer (SCNT) achieved a historic breakthrough in 1996 with the cloning of Dolly the sheep, proving the potential for reprogramming adult cell nuclei to generate entire organisms. Embryonic Stem Cell Transfer gained momentum following the derivation of human embryonic stem cells in 1998, marking a critical advancement in regenerative medicine due to their pluripotency and ability to differentiate into diverse tissue types. These milestones underscore the evolution of cell transfer technologies from cloning to therapeutic applications in biotechnology.
Mechanisms of Somatic Cell Nuclear Transfer
Somatic Cell Nuclear Transfer (SCNT) involves transferring the nucleus of a somatic cell into an enucleated oocyte, reprogramming the somatic nucleus to a totipotent state capable of embryonic development. This process relies on the oocyte's cytoplasmic factors to erase epigenetic marks, enabling the cloned embryo to develop similarly to a naturally fertilized embryo. SCNT contrasts with Embryonic Stem Cell Transfer, which utilizes pluripotent stem cells derived from blastocysts but does not involve nuclear reprogramming of differentiated somatic cells.
Principles of Embryonic Stem Cell Transfer
Embryonic Stem Cell Transfer involves implanting pluripotent stem cells derived from the inner cell mass of a blastocyst into a host embryo to promote tissue regeneration or development. These stem cells maintain the ability to differentiate into various cell types, enabling their use in therapeutic cloning and regenerative medicine. The process relies on precise cell isolation, culture techniques, and integration to ensure proper engraftment and function within the recipient organism.
Efficiency and Success Rates: A Comparative Analysis
Somatic cell nuclear transfer (SCNT) demonstrates higher efficiency in generating patient-specific pluripotent stem cells compared to embryonic stem cell (ESC) transfer due to its direct reprogramming of somatic nuclei, resulting in improved genetic matching and reduced immune rejection. However, ESC transfer generally exhibits higher initial success rates in blastocyst formation and differentiation potential, attributed to the innate pluripotency of ESCs derived from embryos. Comparative studies indicate SCNT faces challenges like lower cloning efficiency and technical complexity, whereas ESC transfer benefits from more established protocols but carries ethical concerns and limited personalized compatibility.
Genetic and Epigenetic Implications
Somatic Cell Nuclear Transfer (SCNT) involves transferring a donor nucleus into an enucleated oocyte, resulting in an organism genetically identical to the donor but with potential epigenetic reprogramming challenges that can affect gene expression patterns and developmental outcomes. In contrast, Embryonic Stem Cell (ESC) transfer utilizes pluripotent cells derived from early embryos, which exhibit a more stable epigenetic landscape but possess genetic variability depending on the embryo source. Both techniques impact DNA methylation, histone modifications, and chromatin remodeling, influencing cellular differentiation and the risk of epigenetic abnormalities that can compromise therapeutic efficacy and genomic integrity.
Therapeutic Applications in Medicine
Somatic Cell Nuclear Transfer (SCNT) enables the creation of patient-specific pluripotent stem cells by transferring a donor nucleus into an enucleated oocyte, offering personalized regenerative therapies without immune rejection risks. Embryonic Stem Cell Transfer involves using pluripotent stem cells derived from embryos to regenerate damaged tissues, but carries ethical concerns and potential immunogenicity when donor and recipient differ. SCNT's capacity for autologous cell generation positions it as a promising tool for treating neurodegenerative diseases, spinal cord injuries, and diabetes through tailored cellular replacement therapies.
Ethical Considerations and Societal Perspectives
Somatic Cell Nuclear Transfer (SCNT) raises ethical concerns due to its cloning implications and potential for human reproductive cloning, challenging existing moral frameworks about individuality and identity. Embryonic Stem Cell Transfer involves the destruction of embryos, which many societies and religious groups oppose on the grounds of human life sanctity and embryo rights. Public opinion on both technologies varies significantly, influenced by cultural, religious, and ethical viewpoints that affect legislative policies and funding for biotechnology research.
Current Challenges and Limitations
Somatic Cell Nuclear Transfer (SCNT) faces significant challenges including low efficiency rates, high incidence of developmental abnormalities, and ethical concerns related to cloning and genetic manipulation. Embryonic Stem Cell (ESC) transfer is limited by immune rejection risks, ethical debates over embryo destruction, and difficulties in directing cell differentiation consistently. Both methods struggle with scalability and clinical translation due to complex technical and regulatory hurdles.
Future Prospects and Innovations in Cell Transfer Technologies
Somatic Cell Nuclear Transfer (SCNT) and Embryonic Stem Cell Transfer each hold transformative potential for regenerative medicine and personalized therapies, with SCNT advancing cloning efficiency and patient-specific cell line creation. Innovations in gene editing tools like CRISPR integrated with SCNT promise enhanced precision in correcting genetic defects before cell transfer. The future of cell transfer technologies lies in optimizing cell viability and differentiation control, enabling scalable production of targeted cell types for disease modeling and therapeutic applications.
Somatic Cell Nuclear Transfer vs Embryonic Stem Cell Transfer Infographic
 techiny.com
techiny.com