Transformation and transfection are key techniques in biotechnology for introducing foreign DNA into cells, with transformation primarily used in bacterial cells and transfection applied to eukaryotic cells. Transformation involves uptake of plasmid DNA through chemical or electrical methods to create genetically modified bacteria, essential for cloning and protein production. Transfection utilizes chemical, lipid-based, or physical methods to deliver nucleic acids into mammalian or other eukaryotic cells, enabling gene expression studies and therapeutic research.
Table of Comparison
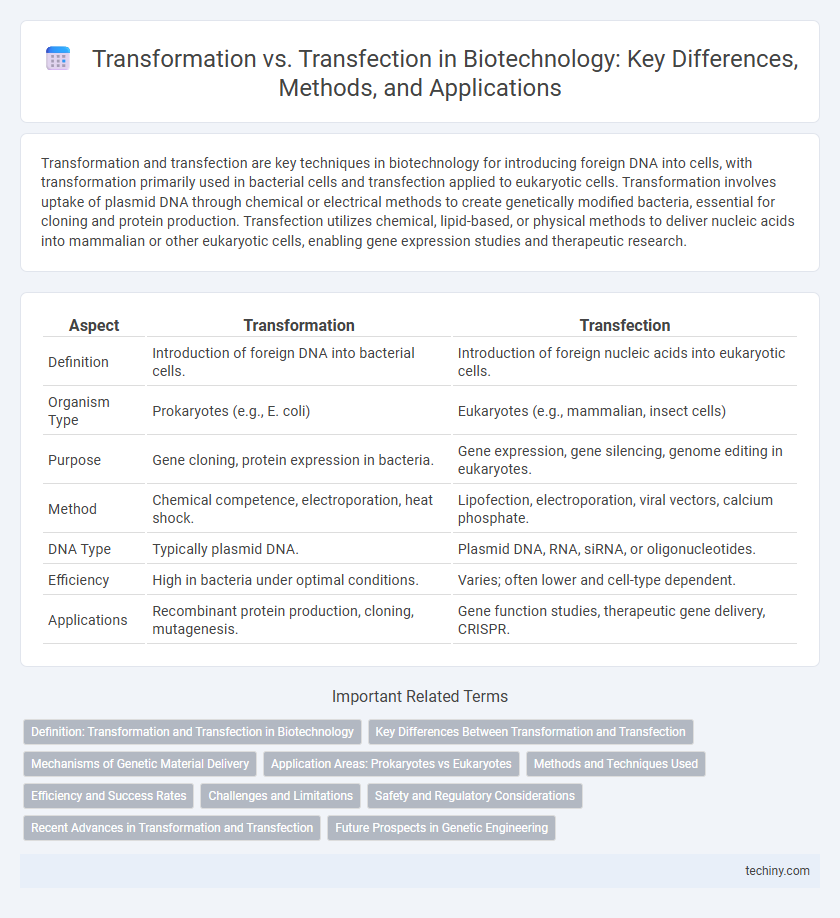
| Aspect | Transformation | Transfection |
|---|---|---|
| Definition | Introduction of foreign DNA into bacterial cells. | Introduction of foreign nucleic acids into eukaryotic cells. |
| Organism Type | Prokaryotes (e.g., E. coli) | Eukaryotes (e.g., mammalian, insect cells) |
| Purpose | Gene cloning, protein expression in bacteria. | Gene expression, gene silencing, genome editing in eukaryotes. |
| Method | Chemical competence, electroporation, heat shock. | Lipofection, electroporation, viral vectors, calcium phosphate. |
| DNA Type | Typically plasmid DNA. | Plasmid DNA, RNA, siRNA, or oligonucleotides. |
| Efficiency | High in bacteria under optimal conditions. | Varies; often lower and cell-type dependent. |
| Applications | Recombinant protein production, cloning, mutagenesis. | Gene function studies, therapeutic gene delivery, CRISPR. |
Definition: Transformation and Transfection in Biotechnology
Transformation in biotechnology refers to the process of introducing foreign DNA into bacterial cells, enabling genetic modification and protein expression. Transfection involves the delivery of nucleic acids, such as DNA or RNA, into eukaryotic cells using chemical, physical, or viral methods to study gene function or produce therapeutic proteins. Both techniques are essential for genetic engineering but target different cell types and utilize distinct mechanisms for nucleic acid uptake.
Key Differences Between Transformation and Transfection
Transformation involves the introduction of foreign DNA into bacterial cells, primarily using chemical or electrical methods to enhance cell membrane permeability, whereas transfection refers to the delivery of nucleic acids into eukaryotic cells using chemical, lipid-based, or viral vectors. Transformation typically results in stable genetic modification with heritable traits, while transfection can be transient or stable depending on the technique and cell type involved. Key differences also include organism targets--prokaryotes for transformation and eukaryotes for transfection--and varying efficiencies influenced by the complexity of the cell membrane and intracellular processing mechanisms.
Mechanisms of Genetic Material Delivery
Transformation involves the uptake of naked DNA by bacterial cells through cell wall permeabilization or chemical treatment, enabling plasmid incorporation for genetic modification. Transfection employs viral vectors, lipid nanoparticles, or electroporation to introduce nucleic acids into eukaryotic cells, facilitating gene expression or silencing. Both processes rely on overcoming cellular barriers, yet differ in delivery efficiency and target cell type specificity.
Application Areas: Prokaryotes vs Eukaryotes
Transformation is primarily used in prokaryotic cells, such as Escherichia coli, for genetic modification and cloning applications, enabling efficient uptake of foreign DNA to study gene function or produce recombinant proteins. Transfection targets eukaryotic cells, including mammalian, plant, and insect cells, facilitating gene expression studies, gene therapy, and development of vaccines by delivering nucleic acids like DNA or RNA into the cell cytoplasm or nucleus. Both techniques play crucial roles in biotechnology, with transformation favored for bacterial systems and transfection optimized for complex eukaryotic cellular models.
Methods and Techniques Used
Transformation in biotechnology primarily involves the uptake of naked DNA by bacterial cells through chemical treatments like calcium chloride and heat shock or electroporation, facilitating genetic modification in prokaryotes. Transfection techniques target eukaryotic cells, employing methods such as lipid-based reagents, calcium phosphate precipitation, electroporation, or viral vectors to introduce nucleic acids efficiently. Both methods depend on optimized protocols to enhance DNA uptake and expression, yet differ in their cellular targets and the complexity of delivery systems.
Efficiency and Success Rates
Transformation in biotechnology typically refers to the uptake of foreign DNA by bacterial cells and generally exhibits higher efficiency and success rates due to the cells' natural competence or chemical/electroporation methods. Transfection, involving the introduction of nucleic acids into eukaryotic cells, tends to have variable efficiency dependent on cell type, transfection reagent, and nucleic acid form, often resulting in lower overall success rates compared to bacterial transformation. Optimizing factors such as cell health, reagent concentration, and incubation conditions directly influences the transfection efficiency, making it crucial for achieving reproducible and high-yield genetic modification in mammalian cell cultures.
Challenges and Limitations
Transformation often faces challenges such as low efficiency in uptaking foreign DNA, especially in certain bacterial strains with restrictive cell walls. Transfection encounters limitations including cytotoxicity and variable expression levels depending on the cell type and transfection reagent used, which can impact experimental reproducibility. Both techniques require optimization of conditions like DNA concentration and cell health to overcome these hurdles and achieve successful gene delivery.
Safety and Regulatory Considerations
Transformation in biotechnology primarily involves introducing DNA into bacterial cells, posing lower biosafety risks and facing less stringent regulatory scrutiny compared to transfection, which often targets eukaryotic cells and can involve viral vectors or chemical reagents with higher biosafety concerns. Transfection methods must comply with rigorous regulations to manage potential cytotoxicity, genetic stability, and biohazard risks, especially in clinical or therapeutic applications. Safety protocols for transfection include containment strategies and extensive validation to ensure minimal off-target effects and environmental release, whereas transformation's simpler, well-characterized procedures generally streamline regulatory approval.
Recent Advances in Transformation and Transfection
Recent advances in biotechnology have significantly enhanced transformation techniques, including the development of novel electroporation and nanoparticle-mediated DNA delivery methods that increase efficiency and cell viability in bacterial and plant cells. In parallel, transfection methods have evolved with CRISPR-based delivery systems and optimized lipid nanoparticles, enabling precise gene editing and transient gene expression in mammalian cells with higher specificity and reduced cytotoxicity. These innovations are driving breakthroughs in genetic engineering, therapeutic development, and synthetic biology applications.
Future Prospects in Genetic Engineering
Future prospects in genetic engineering highlight transformation as a pivotal technique for stable gene integration in bacterial systems, enabling advancements in synthetic biology and metabolic engineering. Transfection, primarily applied to eukaryotic cells, is essential for transient gene expression and precision genome editing using CRISPR-Cas9 technologies, facilitating therapeutic developments. Emerging innovations aim to enhance efficiency and specificity in both methods, accelerating personalized medicine and agricultural biotechnology breakthroughs.
Transformation vs Transfection Infographic
 techiny.com
techiny.com