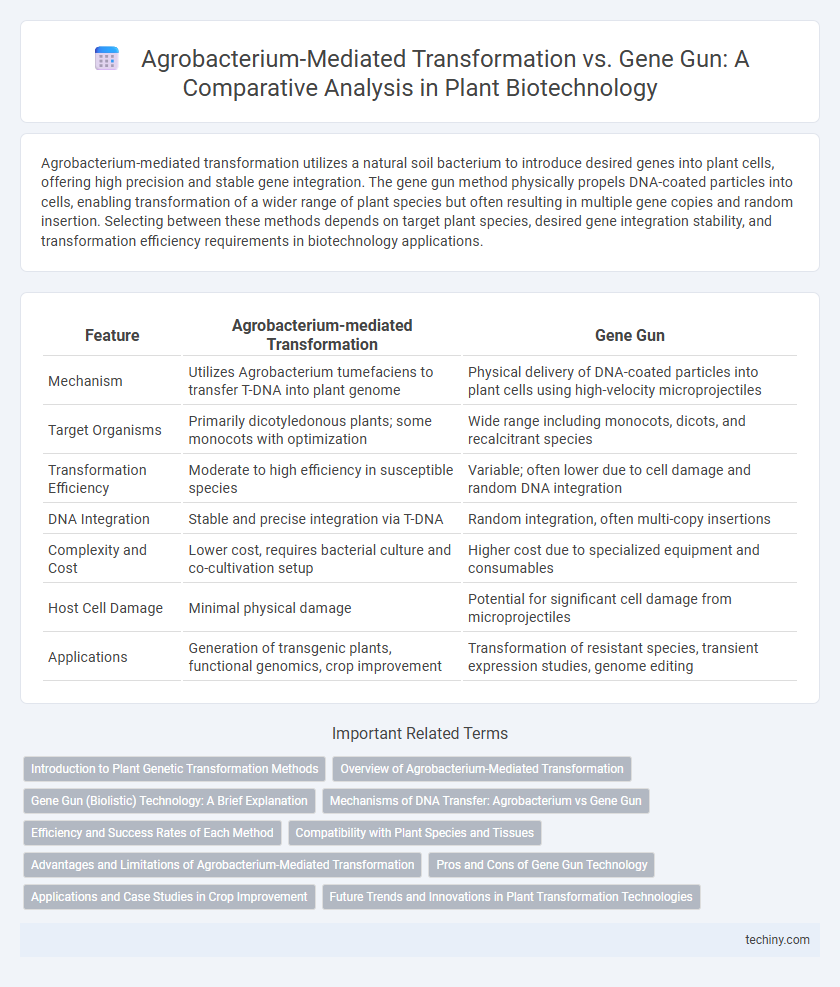
Agrobacterium-mediated transformation utilizes a natural soil bacterium to introduce desired genes into plant cells, offering high precision and stable gene integration. The gene gun method physically propels DNA-coated particles into cells, enabling transformation of a wider range of plant species but often resulting in multiple gene copies and random insertion. Selecting between these methods depends on target plant species, desired gene integration stability, and transformation efficiency requirements in biotechnology applications.
Table of Comparison
| Feature | Agrobacterium-mediated Transformation | Gene Gun |
|---|---|---|
| Mechanism | Utilizes Agrobacterium tumefaciens to transfer T-DNA into plant genome | Physical delivery of DNA-coated particles into plant cells using high-velocity microprojectiles |
| Target Organisms | Primarily dicotyledonous plants; some monocots with optimization | Wide range including monocots, dicots, and recalcitrant species |
| Transformation Efficiency | Moderate to high efficiency in susceptible species | Variable; often lower due to cell damage and random DNA integration |
| DNA Integration | Stable and precise integration via T-DNA | Random integration, often multi-copy insertions |
| Complexity and Cost | Lower cost, requires bacterial culture and co-cultivation setup | Higher cost due to specialized equipment and consumables |
| Host Cell Damage | Minimal physical damage | Potential for significant cell damage from microprojectiles |
| Applications | Generation of transgenic plants, functional genomics, crop improvement | Transformation of resistant species, transient expression studies, genome editing |
Introduction to Plant Genetic Transformation Methods
Agrobacterium-mediated transformation exploits the natural ability of Agrobacterium tumefaciens to transfer T-DNA into plant genomes, offering precise gene integration with minimal copy numbers, making it widely suitable for dicots. In contrast, the gene gun, or biolistic method, physically delivers DNA-coated microprojectiles into plant cells, enabling transformation across a broader range of species including monocots, but often resulting in multiple transgene insertions and possible tissue damage. These complementary techniques have revolutionized plant genetic engineering by enabling targeted manipulation and functional genomics studies in diverse crops.
Overview of Agrobacterium-Mediated Transformation
Agrobacterium-mediated transformation utilizes the natural ability of Agrobacterium tumefaciens to transfer T-DNA into plant genomes, facilitating stable gene integration. This method is highly efficient for dicotyledonous plants and results in low copy number insertions, minimizing gene silencing and ensuring consistent expression. Compared to gene gun techniques, Agrobacterium-mediated transformation offers precise DNA delivery with fewer instances of random integration and cellular damage.
Gene Gun (Biolistic) Technology: A Brief Explanation
Gene Gun (Biolistic) technology delivers DNA-coated microscopic particles directly into plant cells using high-velocity helium gas, enabling genetic modification without the need for bacterial vectors. This method is highly effective for transforming species that are typically resistant to Agrobacterium-mediated transformation, including monocots like maize and wheat. Its precision and versatility make the Gene Gun a valuable tool for both fundamental research and agricultural biotechnology development.
Mechanisms of DNA Transfer: Agrobacterium vs Gene Gun
Agrobacterium-mediated transformation transfers DNA through the natural infection process, where the bacterium inserts a T-DNA segment from its Ti plasmid into the plant genome, enabling stable gene integration. The gene gun employs particle bombardment, physically delivering DNA-coated microprojectiles into plant cells, which can result in multiple and random insertions. Agrobacterium offers targeted and efficient gene transfer mainly in dicotyledonous plants, while the gene gun provides a broader host range but with lower transformation precision.
Efficiency and Success Rates of Each Method
Agrobacterium-mediated transformation demonstrates higher efficiency and success rates in dicotyledonous plants due to its natural ability to transfer T-DNA into plant genomes, resulting in stable gene integration and minimal tissue damage. Gene gun technology offers broader applicability, especially in monocots, but often shows lower transformation efficiency and transient expression with increased tissue damage risks. Optimizing parameters for each method significantly influences transformation success, with Agrobacterium favored for precise gene insertion and gene gun suited for species less susceptible to bacterial infection.
Compatibility with Plant Species and Tissues
Agrobacterium-mediated transformation exhibits high compatibility with dicotyledonous plants and certain monocots, efficiently transferring genes into a variety of tissues, including leaf discs, stems, and roots. The gene gun method, while broadly applicable across numerous plant species and tissue types, is especially valuable for monocots and recalcitrant species where Agrobacterium shows limited effectiveness. Selection between these methods depends on the target plant's susceptibility to Agrobacterium infection and the ability to regenerate transformed tissues, with gene gun providing a versatile alternative for diverse and hard-to-transform species.
Advantages and Limitations of Agrobacterium-Mediated Transformation
Agrobacterium-mediated transformation offers high efficiency in transferring T-DNA into plant genomes, enabling stable gene integration with minimal copy number and low transgene rearrangement. This method is cost-effective and well-suited for dicotyledonous plants, providing precise gene insertion compared to gene gun techniques. However, its limitations include a narrower host range, reduced effectiveness in monocots, and dependency on bacterial infection conditions, restricting its universal application in plant genetic engineering.
Pros and Cons of Gene Gun Technology
Gene gun technology enables direct DNA delivery into plant cells, allowing transformation of a wide range of species without the host specificity limitations seen in Agrobacterium-mediated methods. This physical approach facilitates rapid gene transfer but often results in multiple and random gene insertions, which can complicate downstream genetic analysis and stability. Although versatile, the gene gun's requirement for expensive equipment and potential cellular damage pose challenges compared to the relatively precise and efficient Agrobacterium-mediated transformation.
Applications and Case Studies in Crop Improvement
Agrobacterium-mediated transformation is widely employed in dicotyledonous crops like soybean and tomato, facilitating stable gene integration for traits such as pest resistance and drought tolerance. Gene gun technology, favored for monocots like maize and wheat, enables direct DNA delivery into plant cells, allowing for the introduction of traits like herbicide resistance and enhanced nutritional content. Case studies demonstrate Agrobacterium's efficiency in producing transgenic cotton varieties, while gene gun methods have accelerated improvements in staple cereals by enabling multi-gene insertion events.
Future Trends and Innovations in Plant Transformation Technologies
Agrobacterium-mediated transformation continues to evolve with advancements in CRISPR-Cas systems, enabling more precise and efficient gene editing in diverse plant species. Gene gun technology is seeing innovations such as nanoparticle-based delivery systems that enhance DNA penetration while minimizing tissue damage. Future trends emphasize integrating these methods with synthetic biology and machine learning to accelerate crop improvement and sustainability.
Agrobacterium-mediated Transformation vs Gene Gun Infographic
 techiny.com
techiny.com