Bioreactors and fermenters are essential tools in biotechnology pet applications for cultivating microorganisms or cells under controlled conditions. Bioreactors often provide advanced monitoring and control of parameters such as pH, temperature, and oxygen levels, optimizing cell growth and product yield. Fermenters primarily support microbial fermentation processes, focusing on maintaining anaerobic or aerobic environments essential for producing pet-related biomaterials.
Table of Comparison
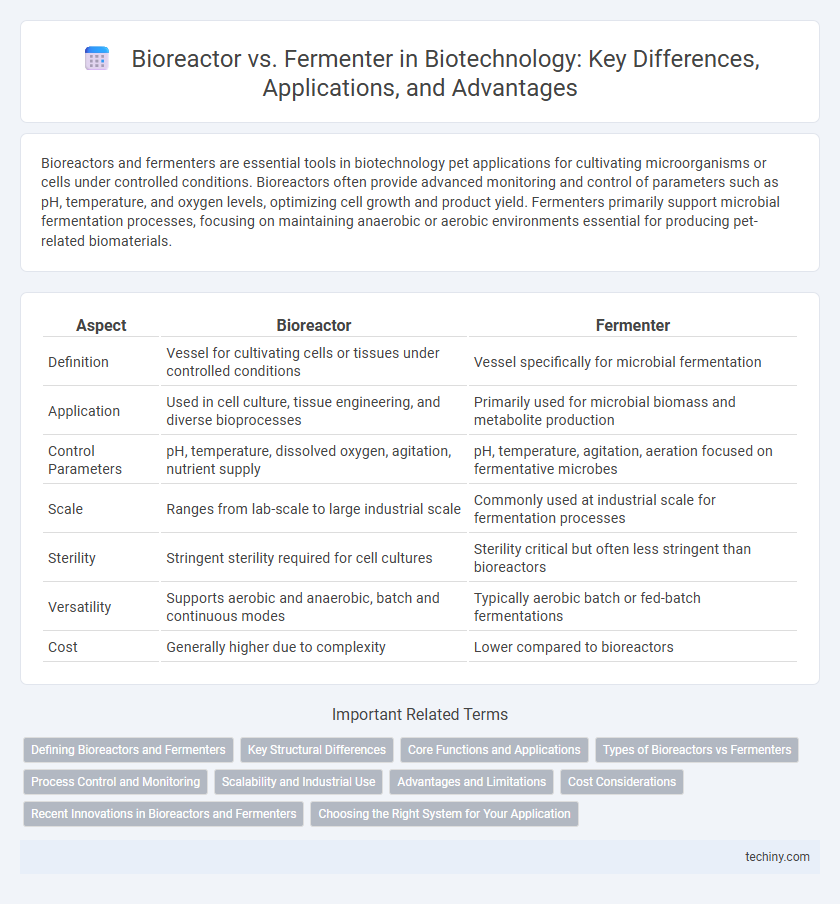
| Aspect | Bioreactor | Fermenter |
|---|---|---|
| Definition | Vessel for cultivating cells or tissues under controlled conditions | Vessel specifically for microbial fermentation |
| Application | Used in cell culture, tissue engineering, and diverse bioprocesses | Primarily used for microbial biomass and metabolite production |
| Control Parameters | pH, temperature, dissolved oxygen, agitation, nutrient supply | pH, temperature, agitation, aeration focused on fermentative microbes |
| Scale | Ranges from lab-scale to large industrial scale | Commonly used at industrial scale for fermentation processes |
| Sterility | Stringent sterility required for cell cultures | Sterility critical but often less stringent than bioreactors |
| Versatility | Supports aerobic and anaerobic, batch and continuous modes | Typically aerobic batch or fed-batch fermentations |
| Cost | Generally higher due to complexity | Lower compared to bioreactors |
Defining Bioreactors and Fermenters
Bioreactors are vessels designed to provide a controlled environment for the growth of microorganisms, plant cells, or animal cells through the regulation of parameters such as temperature, pH, oxygen levels, and agitation. Fermenters are a specific type of bioreactor primarily used for microbial fermentation processes that produce products like antibiotics, alcohol, and enzymes under aerobic or anaerobic conditions. While all fermenters qualify as bioreactors, not all bioreactors are fermenters, as bioreactors also encompass systems for mammalian cell cultures and tissue engineering.
Key Structural Differences
Bioreactors feature advanced control systems for parameters such as pH, temperature, and oxygen levels, enabling precise regulation of microbial or cell cultures, while fermenters primarily support microbial fermentation with simpler control mechanisms. Bioreactors often include specialized components like spargers, baffles, and agitators designed for large-scale cell growth and complex biochemical reactions, contrasted with fermenters, which typically have basic mixing and aeration capabilities. The structural design of bioreactors supports both aerobic and anaerobic processes, whereas fermenters are generally optimized for specific fermentation types, emphasizing scalability and process control differences.
Core Functions and Applications
Bioreactors and fermenters both provide controlled environments for microbial or cell culture growth but differ in design and applications; bioreactors are versatile for aerobic and anaerobic processes and support various cell types including animal, plant, and microbial cultures. Fermenters are specialized bioreactors primarily optimized for microbial fermentation producing products like antibiotics, alcohol, and organic acids. Core functions of bioreactors include maintaining pH, temperature, oxygen levels, and nutrient supply, whereas fermenters focus intensively on maximizing microbial yield and product consistency through agitation and aeration control.
Types of Bioreactors vs Fermenters
Bioreactors encompass a wide range of designs, including stirred-tank, airlift, bubble column, and packed-bed reactors, each optimized for specific biological processes and scale-up requirements. Fermenters, typically a subset of bioreactors, are primarily designed as stirred-tank or batch reactors focused on microbial fermentation and biomass production. The choice between different bioreactor and fermenter types depends on factors like oxygen transfer rates, shear sensitivity, and process control complexity.
Process Control and Monitoring
Bioreactors offer advanced process control and monitoring capabilities, integrating sensors for pH, temperature, dissolved oxygen, and agitation speed to optimize cell growth and product yield. Fermenters typically provide basic monitoring focused on fermentation parameters with limited automation, suitable for simpler microbial cultures. Enhanced real-time data acquisition in bioreactors enables dynamic adjustments, improving efficiency and reproducibility in complex bioprocesses.
Scalability and Industrial Use
Bioreactors offer enhanced scalability for industrial biotechnology applications due to their advanced control over environmental parameters such as pH, temperature, and oxygen levels, making them suitable for large-scale production. Fermenters, traditionally used for microbial fermentation, are typically limited by design to smaller volumes and less precise control, which can restrict their efficiency in scaling up processes. The ability of bioreactors to integrate automation and real-time monitoring supports consistent product quality and higher yield in industrial fermentation and cell culture operations.
Advantages and Limitations
Bioreactors offer precise control over environmental parameters such as pH, temperature, and oxygen concentration, facilitating optimal growth conditions for diverse biological processes, while fermenters generally provide simpler, cost-effective setups suitable for large-scale microbial fermentation. Advantages of bioreactors include the ability to support both aerobic and anaerobic cultures with enhanced monitoring systems, whereas fermenters may face limitations in scalability and process control flexibility. Despite their complexity and higher operational costs, bioreactors enable advanced bioprocessing applications, whereas fermenters remain favored for traditional fermentation tasks due to ease of use and lower maintenance requirements.
Cost Considerations
Bioreactors generally involve higher initial capital investment due to advanced design features and automation for precise control, while fermenters often cost less and are suited for simpler microbial processes. Operational expenses for bioreactors can be lower over time because of improved efficiency, scalability, and reduced contamination risks compared to traditional fermenters. Cost-effectiveness depends on the specific application, with bioreactors favored for complex, high-value products and fermenters preferred for large-scale, low-cost production.
Recent Innovations in Bioreactors and Fermenters
Recent innovations in bioreactors and fermenters have focused on enhancing process control and scalability through advanced sensors and automation technology. Integration of real-time monitoring systems using IoT and AI enables precise regulation of parameters such as pH, temperature, and oxygen levels, optimizing microbial growth and product yield. These developments improve bioprocess efficiency in pharmaceutical, agricultural, and industrial biotechnology applications.
Choosing the Right System for Your Application
Selecting the appropriate system between a bioreactor and a fermenter depends on the specific biotechnological application and operational requirements. Bioreactors offer precise control over environmental parameters like pH, temperature, and oxygen levels, making them ideal for cell culture and complex biochemical processes. Fermenters are optimized for microbial growth and large-scale production of metabolites, emphasizing robust mixing and aeration for efficient fermentation.
Bioreactor vs Fermenter Infographic
 techiny.com
techiny.com