Directed evolution accelerates protein optimization by mimicking natural selection, generating diverse variants and selecting those with desired traits in biotechnology pet applications. Rational design relies on detailed knowledge of protein structure and function to engineer specific modifications, offering precision but limited by current scientific understanding. Balancing these approaches enhances the development of innovative biotechnological solutions for pet health and nutrition.
Table of Comparison
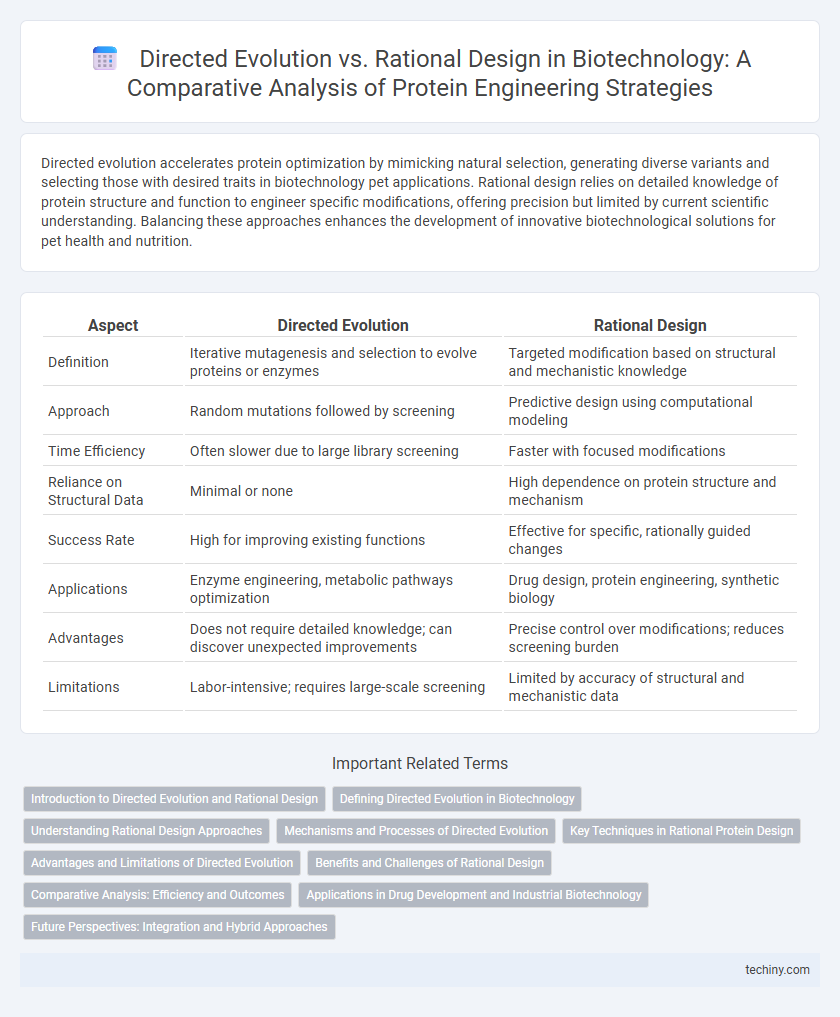
| Aspect | Directed Evolution | Rational Design |
|---|---|---|
| Definition | Iterative mutagenesis and selection to evolve proteins or enzymes | Targeted modification based on structural and mechanistic knowledge |
| Approach | Random mutations followed by screening | Predictive design using computational modeling |
| Time Efficiency | Often slower due to large library screening | Faster with focused modifications |
| Reliance on Structural Data | Minimal or none | High dependence on protein structure and mechanism |
| Success Rate | High for improving existing functions | Effective for specific, rationally guided changes |
| Applications | Enzyme engineering, metabolic pathways optimization | Drug design, protein engineering, synthetic biology |
| Advantages | Does not require detailed knowledge; can discover unexpected improvements | Precise control over modifications; reduces screening burden |
| Limitations | Labor-intensive; requires large-scale screening | Limited by accuracy of structural and mechanistic data |
Introduction to Directed Evolution and Rational Design
Directed evolution mimics natural selection by generating genetic diversity and screening for desired traits, accelerating enzyme optimization without prior structural knowledge. Rational design relies on detailed understanding of molecular structures to introduce specific mutations, enabling targeted protein engineering based on computational models. Both methods enhance protein functionality, but directed evolution excels in exploring vast sequence space, while rational design offers precision guided by structural insights.
Defining Directed Evolution in Biotechnology
Directed evolution in biotechnology is a powerful method that mimics natural selection to evolve proteins or nucleic acids toward a user-defined goal by iterative rounds of mutation and selection. This technique enables the rapid optimization of enzyme functions, binding affinities, or stability without detailed knowledge of the molecular structure. Directed evolution accelerates the development of biocatalysts and therapeutic agents, offering a versatile alternative to rational design strategies that rely on computational modeling and structural prediction.
Understanding Rational Design Approaches
Rational design approaches in biotechnology leverage detailed knowledge of protein structure and function to engineer specific modifications at the molecular level. Computational modeling and site-directed mutagenesis enable precise alterations, targeting active sites or stabilizing regions to enhance enzyme performance or binding affinity. This method contrasts with directed evolution by relying on predictive frameworks rather than iterative screening of random mutations.
Mechanisms and Processes of Directed Evolution
Directed evolution mimics natural selection by generating a diverse library of genetic variants through random mutagenesis or recombination, followed by iterative rounds of selection or screening to isolate desired traits. This process leverages high-throughput assays to identify improved protein functions without prior structural knowledge, enabling adaptation to new substrates or enhanced stability. Unlike rational design, directed evolution relies on empirical selection mechanisms rather than computational predictions, facilitating rapid optimization of biomolecules in complex biological systems.
Key Techniques in Rational Protein Design
Key techniques in rational protein design include computational modeling, site-directed mutagenesis, and structure-based design, enabling precise alterations in protein sequences to enhance function or stability. High-resolution crystallography and molecular dynamics simulations provide critical structural insights that guide targeted modifications. These methods accelerate the development of proteins with desired catalytic properties or binding affinities, contrasting with the iterative, random nature of directed evolution.
Advantages and Limitations of Directed Evolution
Directed evolution offers the advantage of generating novel protein functions without requiring detailed structural knowledge, leveraging iterative rounds of mutation and selection to mimic natural selection processes. This approach can overcome complex fitness landscapes and discover unexpected beneficial mutations but often requires extensive screening and can be time-consuming and resource-intensive. Limitations include reduced efficiency for proteins with inherently low mutational tolerance and difficulty in directing evolution toward specific, predetermined functional outcomes compared to rational design.
Benefits and Challenges of Rational Design
Rational design in biotechnology enables precise protein engineering by using computational models and structural data to predict and implement specific amino acid changes, enhancing enzyme specificity and stability. Its benefits include targeted modifications and increased efficiency in developing biomolecules with desired functions, reducing trial-and-error experimentation. However, challenges involve the complexity of accurately modeling protein folding and dynamics, often requiring extensive computational resources and expertise to achieve successful outcomes.
Comparative Analysis: Efficiency and Outcomes
Directed evolution accelerates enzyme optimization by mimicking natural selection through iterative genetic diversification and screening, often yielding highly efficient variants without prior structural knowledge. Rational design relies on detailed understanding of protein structure and function, enabling targeted modifications but is limited by the accuracy of computational models and available structural data. Comparative analysis reveals directed evolution excels in discovering novel functionalities with robust performance, while rational design offers precision and mechanistic insights, making a hybrid approach increasingly favored for maximizing biocatalyst efficiency and desired outcomes.
Applications in Drug Development and Industrial Biotechnology
Directed evolution accelerates enzyme and protein optimization by mimicking natural selection, making it ideal for drug development to enhance therapeutic efficacy and reduce side effects. Rational design utilizes computational models to predict molecular modifications, enabling precise engineering of biomolecules for industrial biotechnology applications such as biofuel production and biodegradable plastics. Combining both techniques drives innovation in creating novel pharmaceuticals and sustainable industrial biocatalysts with improved performance and specificity.
Future Perspectives: Integration and Hybrid Approaches
Future perspectives in biotechnology emphasize the integration of Directed Evolution and Rational Design to enhance enzyme engineering and protein optimization. Hybrid approaches combine the iterative mutation and selection cycles of Directed Evolution with the predictive power of computational Rational Design, accelerating the development of biomolecules with improved functionality and stability. This synergy leverages high-throughput screening technologies and machine learning algorithms to expand the scope of synthetic biology applications in medicine, agriculture, and industrial biocatalysis.
Directed Evolution vs Rational Design Infographic
 techiny.com
techiny.com