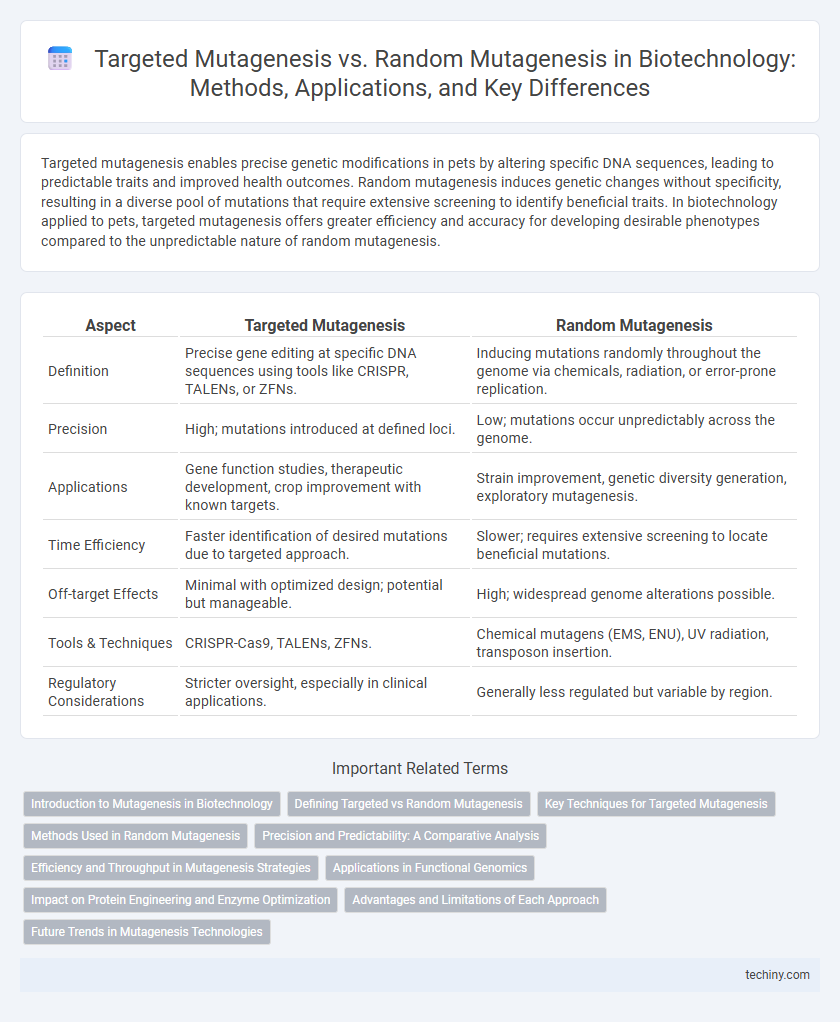
Targeted mutagenesis enables precise genetic modifications in pets by altering specific DNA sequences, leading to predictable traits and improved health outcomes. Random mutagenesis induces genetic changes without specificity, resulting in a diverse pool of mutations that require extensive screening to identify beneficial traits. In biotechnology applied to pets, targeted mutagenesis offers greater efficiency and accuracy for developing desirable phenotypes compared to the unpredictable nature of random mutagenesis.
Table of Comparison
| Aspect | Targeted Mutagenesis | Random Mutagenesis |
|---|---|---|
| Definition | Precise gene editing at specific DNA sequences using tools like CRISPR, TALENs, or ZFNs. | Inducing mutations randomly throughout the genome via chemicals, radiation, or error-prone replication. |
| Precision | High; mutations introduced at defined loci. | Low; mutations occur unpredictably across the genome. |
| Applications | Gene function studies, therapeutic development, crop improvement with known targets. | Strain improvement, genetic diversity generation, exploratory mutagenesis. |
| Time Efficiency | Faster identification of desired mutations due to targeted approach. | Slower; requires extensive screening to locate beneficial mutations. |
| Off-target Effects | Minimal with optimized design; potential but manageable. | High; widespread genome alterations possible. |
| Tools & Techniques | CRISPR-Cas9, TALENs, ZFNs. | Chemical mutagens (EMS, ENU), UV radiation, transposon insertion. |
| Regulatory Considerations | Stricter oversight, especially in clinical applications. | Generally less regulated but variable by region. |
Introduction to Mutagenesis in Biotechnology
Targeted mutagenesis enables precise alterations in specific DNA sequences, enhancing gene function studies and therapeutic development with high accuracy. Random mutagenesis introduces mutations throughout the genome, generating diverse genetic variants for screening without prior knowledge of gene function. Both methods are fundamental in biotechnology for advancing genetic engineering, strain improvement, and functional genomics.
Defining Targeted vs Random Mutagenesis
Targeted mutagenesis involves precise, intentional alterations at specific genomic locations using tools like CRISPR-Cas9 or TALENs, enabling controlled genetic modifications. Random mutagenesis generates mutations unpredictably throughout the genome via physical, chemical, or biological agents, creating diverse genetic variations without predetermined sites. These differing approaches impact experimental outcomes by offering either specificity and efficiency in gene editing or broad mutation spectra for evolutionary studies and strain improvement.
Key Techniques for Targeted Mutagenesis
Key techniques for targeted mutagenesis in biotechnology include CRISPR-Cas9, TALENs, and zinc-finger nucleases, which enable precise gene editing by creating double-strand breaks at specific DNA sequences. Base editors and prime editors further enhance specificity by allowing direct nucleotide conversions without inducing double-strand breaks. These methods improve accuracy and efficiency in generating desired mutations compared to random mutagenesis, which relies on non-specific chemical or physical mutagens.
Methods Used in Random Mutagenesis
Random mutagenesis employs techniques such as chemical mutagens, ultraviolet (UV) irradiation, and error-prone PCR to introduce mutations throughout the genome without sequence specificity. Chemical agents like ethyl methanesulfonate (EMS) induce nucleotide substitutions by alkylating DNA bases, while UV light generates thymine dimers leading to replication errors. Error-prone PCR increases mutation rates by using low-fidelity DNA polymerases, enabling diverse genetic variants for screening in strain improvement and functional genomics studies.
Precision and Predictability: A Comparative Analysis
Targeted mutagenesis offers high precision by introducing specific genetic changes at predetermined sites using tools like CRISPR-Cas9, enabling predictable outcomes in genetic modifications. In contrast, random mutagenesis induces mutations throughout the genome without specificity, resulting in unpredictable phenotypic variations that require extensive screening. The enhanced predictability and precision of targeted mutagenesis significantly accelerate functional genomics studies and therapeutic gene editing applications.
Efficiency and Throughput in Mutagenesis Strategies
Targeted mutagenesis offers higher efficiency by precisely editing specific genomic sites, reducing off-target effects and accelerating functional validation in biotechnology applications. Random mutagenesis generates extensive genetic diversity, enabling high-throughput screening for novel phenotypes but requires more extensive downstream analysis to identify beneficial mutations. Advances in CRISPR-Cas systems and directed evolution techniques have enhanced both strategies, balancing precision and diversity to optimize mutagenesis workflows.
Applications in Functional Genomics
Targeted mutagenesis enables precise alterations in specific genes, facilitating detailed functional analyses and the validation of gene function in model organisms and cell lines. Random mutagenesis generates diverse mutations across the genome, providing a broad, unbiased approach to identify novel gene functions and genetic interactions. Functional genomics leverages both methods to dissect complex biological pathways and accelerate gene discovery in systems biology and therapeutic development.
Impact on Protein Engineering and Enzyme Optimization
Targeted mutagenesis enables precise alterations in specific amino acid residues, significantly enhancing protein engineering by allowing systematic exploration of structure-function relationships and improving enzyme specificity, stability, and activity. Random mutagenesis generates diverse genetic variants, facilitating the discovery of novel enzyme functions through high-throughput screening, but with less control over mutation sites. Combining both approaches accelerates enzyme optimization by balancing targeted modifications with broad diversity to achieve desired catalytic properties.
Advantages and Limitations of Each Approach
Targeted mutagenesis allows precise genetic modifications by directly altering specific DNA sequences, offering greater control and predictability in gene function studies and therapeutic applications, but it requires detailed genetic information and complex tools like CRISPR-Cas9. Random mutagenesis generates diverse mutations across the genome without prior knowledge of target sites, facilitating the discovery of novel gene functions or traits but often necessitates extensive screening to identify desirable mutants. Both approaches complement each other in biotechnology research, with targeted mutagenesis excelling in specificity and random mutagenesis providing broad genetic diversity.
Future Trends in Mutagenesis Technologies
Future trends in mutagenesis technologies emphasize the increasing precision and efficiency of targeted mutagenesis methods, such as CRISPR-Cas systems, which allow for specific gene editing with minimal off-target effects. Advancements in high-throughput sequencing and machine learning algorithms enhance the prediction and design of beneficial mutations, accelerating strain optimization for industrial biotechnology applications. Despite ongoing improvements in random mutagenesis techniques, the growing preference for targeted approaches reflects their superior control and potential for sustainable, tailored genetic modifications.
targeted mutagenesis vs random mutagenesis Infographic
 techiny.com
techiny.com