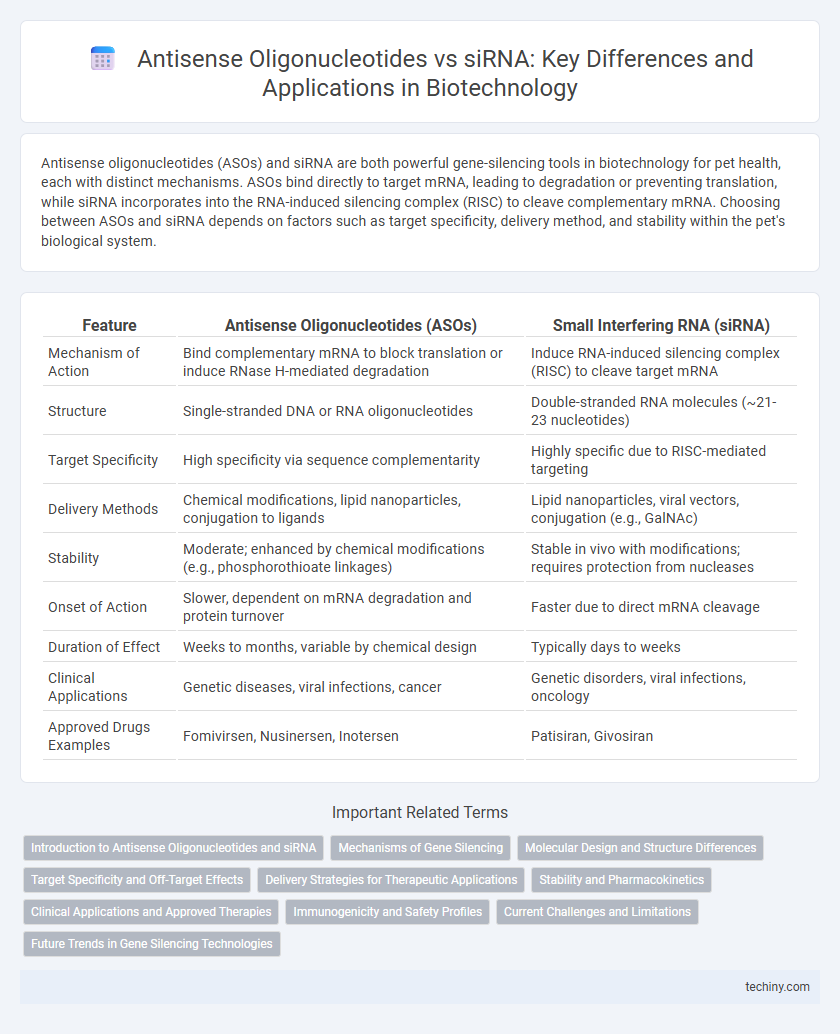
Antisense oligonucleotides (ASOs) and siRNA are both powerful gene-silencing tools in biotechnology for pet health, each with distinct mechanisms. ASOs bind directly to target mRNA, leading to degradation or preventing translation, while siRNA incorporates into the RNA-induced silencing complex (RISC) to cleave complementary mRNA. Choosing between ASOs and siRNA depends on factors such as target specificity, delivery method, and stability within the pet's biological system.
Table of Comparison
| Feature | Antisense Oligonucleotides (ASOs) | Small Interfering RNA (siRNA) |
|---|---|---|
| Mechanism of Action | Bind complementary mRNA to block translation or induce RNase H-mediated degradation | Induce RNA-induced silencing complex (RISC) to cleave target mRNA |
| Structure | Single-stranded DNA or RNA oligonucleotides | Double-stranded RNA molecules (~21-23 nucleotides) |
| Target Specificity | High specificity via sequence complementarity | Highly specific due to RISC-mediated targeting |
| Delivery Methods | Chemical modifications, lipid nanoparticles, conjugation to ligands | Lipid nanoparticles, viral vectors, conjugation (e.g., GalNAc) |
| Stability | Moderate; enhanced by chemical modifications (e.g., phosphorothioate linkages) | Stable in vivo with modifications; requires protection from nucleases |
| Onset of Action | Slower, dependent on mRNA degradation and protein turnover | Faster due to direct mRNA cleavage |
| Duration of Effect | Weeks to months, variable by chemical design | Typically days to weeks |
| Clinical Applications | Genetic diseases, viral infections, cancer | Genetic disorders, viral infections, oncology |
| Approved Drugs Examples | Fomivirsen, Nusinersen, Inotersen | Patisiran, Givosiran |
Introduction to Antisense Oligonucleotides and siRNA
Antisense oligonucleotides (ASOs) are short, synthetic strands of nucleotides designed to bind specifically to target mRNA sequences, inhibiting gene expression through RNase H-mediated degradation or splicing modulation. Small interfering RNA (siRNA) is a class of double-stranded RNA molecules that induce gene silencing by guiding the RNA-induced silencing complex (RISC) to degrade complementary mRNA. Both ASOs and siRNA serve as powerful tools in gene therapy and molecular biology for precise regulation of gene expression.
Mechanisms of Gene Silencing
Antisense oligonucleotides (ASOs) silence genes by binding complementary mRNA sequences, inducing RNase H-mediated degradation or blocking translation. Small interfering RNAs (siRNAs) incorporate into the RNA-induced silencing complex (RISC) to guide cleavage of target mRNA through perfect base pairing. Both ASOs and siRNAs achieve post-transcriptional gene silencing but differ in cellular pathways and molecular interactions.
Molecular Design and Structure Differences
Antisense oligonucleotides (ASOs) are single-stranded DNA or RNA molecules designed to bind complementary mRNA sequences, primarily modulating gene expression through steric blocking or RNase H-mediated degradation. Small interfering RNA (siRNA) consists of double-stranded RNA duplexes, usually 20-25 nucleotides long, functioning within the RNA-induced silencing complex (RISC) to induce sequence-specific mRNA cleavage. Key structural differences lie in ASOs' single-stranded nature enabling direct hybridization to target mRNA, whereas siRNAs require duplex formation and unwinding to guide mRNA degradation via the RISC pathway.
Target Specificity and Off-Target Effects
Antisense oligonucleotides (ASOs) exhibit high target specificity by binding complementary mRNA sequences, enabling precise gene silencing with reduced off-target effects compared to siRNA, which can sometimes induce unintended gene silencing due to partial sequence homology. siRNA mechanisms rely on RNA-induced silencing complex (RISC), which enhances gene knockdown efficacy but increases the risk of off-target interactions and immune activation. Advances in chemical modifications for ASOs improve their stability and target affinity, further minimizing off-target molecular interactions and enhancing therapeutic safety profiles.
Delivery Strategies for Therapeutic Applications
Antisense oligonucleotides (ASOs) and siRNA both require sophisticated delivery strategies to overcome cellular barriers and achieve effective therapeutic outcomes, often utilizing lipid nanoparticles, conjugation with cell-penetrating peptides, or polymer-based carriers to enhance stability and target specificity. ASOs commonly benefit from chemical modifications such as phosphorothioate backbones to improve nuclease resistance and tissue distribution, while siRNA delivery frequently relies on encapsulation in lipid nanoparticles to protect against degradation and facilitate endosomal escape. Targeted delivery systems employing ligand-receptor interactions, such as GalNAc conjugation for liver-specific uptake, represent a critical advancement in improving the precision and efficacy of both antisense and siRNA therapeutics.
Stability and Pharmacokinetics
Antisense oligonucleotides (ASOs) typically exhibit enhanced stability in biological systems due to chemical modifications such as phosphorothioate backbones, which improve resistance to nuclease degradation. Small interfering RNA (siRNA) molecules often require encapsulation in lipid nanoparticles or conjugation to improve serum stability and cellular uptake. Pharmacokinetically, ASOs generally demonstrate longer half-lives and prolonged tissue retention compared to siRNAs, enabling less frequent dosing in therapeutic applications.
Clinical Applications and Approved Therapies
Antisense oligonucleotides (ASOs) and siRNA both target mRNA to modulate gene expression, but ASOs have a broader range of clinical applications including treatment of spinal muscular atrophy and Duchenne muscular dystrophy through FDA-approved therapies like Spinraza and Exondys 51. siRNA therapies, such as Onpattro and Givlaari, have been successfully approved for rare genetic disorders like hereditary transthyretin amyloidosis and acute hepatic porphyria, highlighting their precision in silencing specific gene targets. The distinct pharmacokinetic profiles and delivery mechanisms of ASOs and siRNA influence their therapeutic use in gene-based disease interventions.
Immunogenicity and Safety Profiles
Antisense oligonucleotides (ASOs) generally exhibit lower immunogenicity compared to siRNA due to their single-stranded DNA or RNA design, which elicits minimal innate immune responses. siRNA can activate Toll-like receptors (TLRs), particularly TLR7 and TLR8, leading to stronger immune activation and potential cytokine release syndrome. Safety profiles of ASOs often demonstrate reduced off-target effects and long-term tolerability, whereas siRNA treatments require careful modification to mitigate immunostimulation and enhance biocompatibility.
Current Challenges and Limitations
Antisense oligonucleotides (ASOs) face challenges such as limited cellular uptake, stability issues, and off-target effects that hinder therapeutic efficacy. Small interfering RNA (siRNA) struggles with delivery barriers, immune activation, and endosomal escape that reduce gene silencing efficiency. Both technologies require improved delivery systems and chemical modifications to overcome degradation and enhance target specificity in clinical applications.
Future Trends in Gene Silencing Technologies
Antisense oligonucleotides (ASOs) and small interfering RNA (siRNA) represent pivotal gene silencing technologies with distinct mechanisms influencing future therapeutic applications. Advancements in chemical modifications and delivery systems are driving enhanced stability and targeted delivery for both ASOs and siRNA, propelling their use in treating genetic disorders and cancers. Emerging trends emphasize personalized medicine and combination therapies, leveraging the complementary strengths of ASOs and siRNA to achieve precise and durable gene suppression.
antisense oligonucleotides vs siRNA Infographic
 techiny.com
techiny.com