FRET (Fluorescence Resonance Energy Transfer) and BRET (Bioluminescence Resonance Energy Transfer) are essential techniques in biotechnology for studying protein interactions and cellular processes within pets. FRET relies on external light excitation to transfer energy between fluorophores, while BRET utilizes bioluminescent proteins that generate light without external excitation, reducing background noise and improving signal specificity. Choosing between FRET and BRET depends on experimental conditions, with BRET often preferred for live-cell imaging due to its higher sensitivity and lower phototoxicity.
Table of Comparison
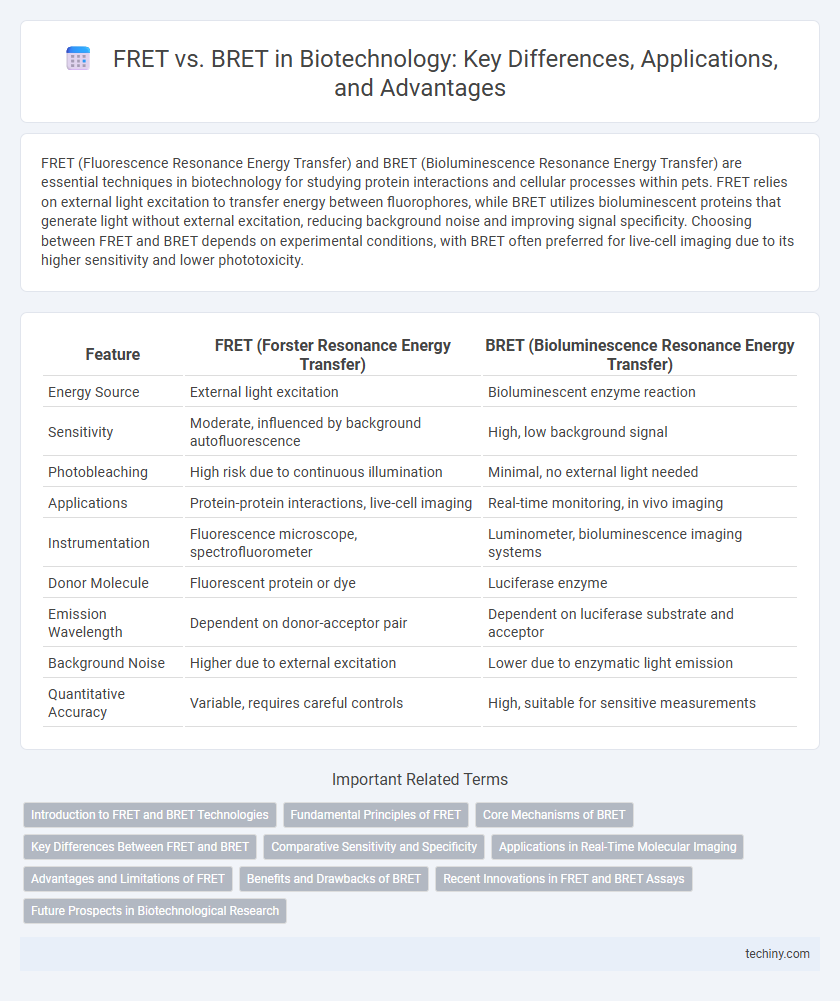
| Feature | FRET (Forster Resonance Energy Transfer) | BRET (Bioluminescence Resonance Energy Transfer) |
|---|---|---|
| Energy Source | External light excitation | Bioluminescent enzyme reaction |
| Sensitivity | Moderate, influenced by background autofluorescence | High, low background signal |
| Photobleaching | High risk due to continuous illumination | Minimal, no external light needed |
| Applications | Protein-protein interactions, live-cell imaging | Real-time monitoring, in vivo imaging |
| Instrumentation | Fluorescence microscope, spectrofluorometer | Luminometer, bioluminescence imaging systems |
| Donor Molecule | Fluorescent protein or dye | Luciferase enzyme |
| Emission Wavelength | Dependent on donor-acceptor pair | Dependent on luciferase substrate and acceptor |
| Background Noise | Higher due to external excitation | Lower due to enzymatic light emission |
| Quantitative Accuracy | Variable, requires careful controls | High, suitable for sensitive measurements |
Introduction to FRET and BRET Technologies
FRET (Forster Resonance Energy Transfer) and BRET (Bioluminescence Resonance Energy Transfer) are powerful biotechnological techniques used to study molecular interactions by measuring energy transfer between donor and acceptor molecules. FRET relies on excitation of a fluorescent donor molecule, transferring energy to a fluorescent acceptor, while BRET employs a bioluminescent enzyme as the donor, eliminating the need for external light excitation and reducing background noise. Both technologies enable real-time, non-invasive monitoring of protein-protein interactions, conformational changes, and cellular signaling pathways with high spatial and temporal resolution.
Fundamental Principles of FRET
Fluorescence Resonance Energy Transfer (FRET) operates on the fundamental principle of non-radiative energy transfer between a donor fluorophore and an acceptor fluorophore when in close proximity, typically 1-10 nanometers. This distance-dependent interaction allows researchers to monitor molecular interactions and conformational changes with high spatial resolution. In biotechnology, FRET is extensively utilized for real-time visualization of protein-protein interactions, enabling detailed studies of cellular signaling pathways.
Core Mechanisms of BRET
BRET (Bioluminescence Resonance Energy Transfer) operates through the energy transfer from a bioluminescent donor enzyme, commonly Renilla luciferase, to a fluorescent acceptor molecule without an external light source, enabling sensitive detection of molecular interactions in live cells. This core mechanism relies on the enzymatic oxidation of a substrate by the luciferase to produce light, which then non-radiatively transfers energy to the acceptor fluorophore if in close proximity, typically within 1-10 nm. The absence of external excitation reduces background fluorescence and phototoxicity, making BRET particularly advantageous for real-time monitoring of protein-protein interactions and conformational changes in complex biological systems.
Key Differences Between FRET and BRET
FRET (Forster Resonance Energy Transfer) relies on a fluorescent donor and acceptor pair, requiring external light excitation, while BRET (Bioluminescence Resonance Energy Transfer) uses a bioluminescent enzyme donor that generates light through a chemical reaction, eliminating the need for external illumination. FRET exhibits higher background noise due to autofluorescence and photobleaching, whereas BRET offers lower background with improved signal stability, making it ideal for live-cell and in vivo imaging. The energy transfer efficiency and spectral overlap differ, with FRET demanding closer proximity and specific spectral overlap, while BRET allows broader spectral combinations and enhanced sensitivity in detecting protein-protein interactions.
Comparative Sensitivity and Specificity
FRET (Forster Resonance Energy Transfer) and BRET (Bioluminescence Resonance Energy Transfer) differ significantly in sensitivity and specificity within biotechnological applications. FRET relies on external light excitation, often resulting in higher background fluorescence and reduced sensitivity, whereas BRET utilizes bioluminescent proteins that generate light intrinsically, leading to lower background noise and enhanced detection sensitivity. Specificity in BRET surpasses FRET due to its reduced photobleaching and minimized autofluorescence, making BRET particularly advantageous for real-time, live-cell imaging and protein-protein interaction studies.
Applications in Real-Time Molecular Imaging
FRET (Forster Resonance Energy Transfer) and BRET (Bioluminescence Resonance Energy Transfer) enable real-time molecular imaging by detecting protein-protein interactions and conformational changes with high spatial and temporal resolution. FRET relies on fluorescent donor and acceptor pairs, ideal for live-cell imaging and monitoring dynamic cellular processes, whereas BRET uses a bioluminescent donor, eliminating the need for external excitation and reducing background autofluorescence in deep tissue imaging. Both techniques play crucial roles in drug discovery, signal transduction studies, and understanding molecular mechanisms in complex biological systems.
Advantages and Limitations of FRET
FRET (Forster Resonance Energy Transfer) offers high spatial resolution for detecting molecular interactions at nanometer distances, making it ideal for studying protein-protein interactions and conformational changes in live cells. Its limitations include sensitivity to photobleaching, autofluorescence background interference, and a requirement for precise donor-acceptor spectral overlap, which can restrict experimental flexibility. Despite these challenges, FRET remains a powerful tool for real-time dynamic analysis in cell biology and biotechnology applications.
Benefits and Drawbacks of BRET
BRET (bioluminescence resonance energy transfer) offers the benefit of reduced background signal due to the absence of external light excitation, enhancing sensitivity in live-cell imaging compared to FRET (fluorescence resonance energy transfer). BRET enables real-time monitoring of protein-protein interactions with minimal phototoxicity and photobleaching, making it ideal for dynamic studies in physiological conditions. However, its drawbacks include a limited range of available donor-acceptor pairs and typically lower signal intensity, which can restrict multiplexing capabilities and assay versatility.
Recent Innovations in FRET and BRET Assays
Recent innovations in FRET and BRET assays have enhanced sensitivity and expanded multiplexing capabilities, enabling real-time monitoring of complex biomolecular interactions with higher spatial and temporal resolution. Advanced bioluminescent proteins and novel fluorescent probes have improved signal-to-noise ratios, facilitating applications in live-cell imaging and high-throughput screening. Integration of artificial intelligence algorithms further optimizes data analysis, accelerating drug discovery and precision medicine research in biotechnology.
Future Prospects in Biotechnological Research
FRET (Forster Resonance Energy Transfer) and BRET (Bioluminescence Resonance Energy Transfer) offer complementary advantages for real-time, non-invasive biomolecular interaction studies, with BRET showing promise for in vivo applications due to its lower background signal and higher sensitivity. Advances in protein engineering and synthetic biology are expanding the range of donor-acceptor pairs, enhancing multiplexing capabilities and improving spatial-temporal resolution in complex biological systems. Integration of FRET and BRET with cutting-edge imaging technologies is expected to drive breakthroughs in drug discovery, cellular signaling analysis, and early disease diagnosis.
FRET vs BRET Infographic
 techiny.com
techiny.com