Real-time PCR (qPCR) provides quantitative data by measuring fluorescence during the amplification process, making it ideal for detecting and quantifying nucleic acids in pet biotechnology applications. Digital PCR (dPCR) offers higher precision by partitioning samples into thousands of individual reactions, enabling absolute quantification without the need for standard curves. In pet biotechnology, dPCR enhances sensitivity and accuracy for genetic testing and pathogen detection compared to qPCR.
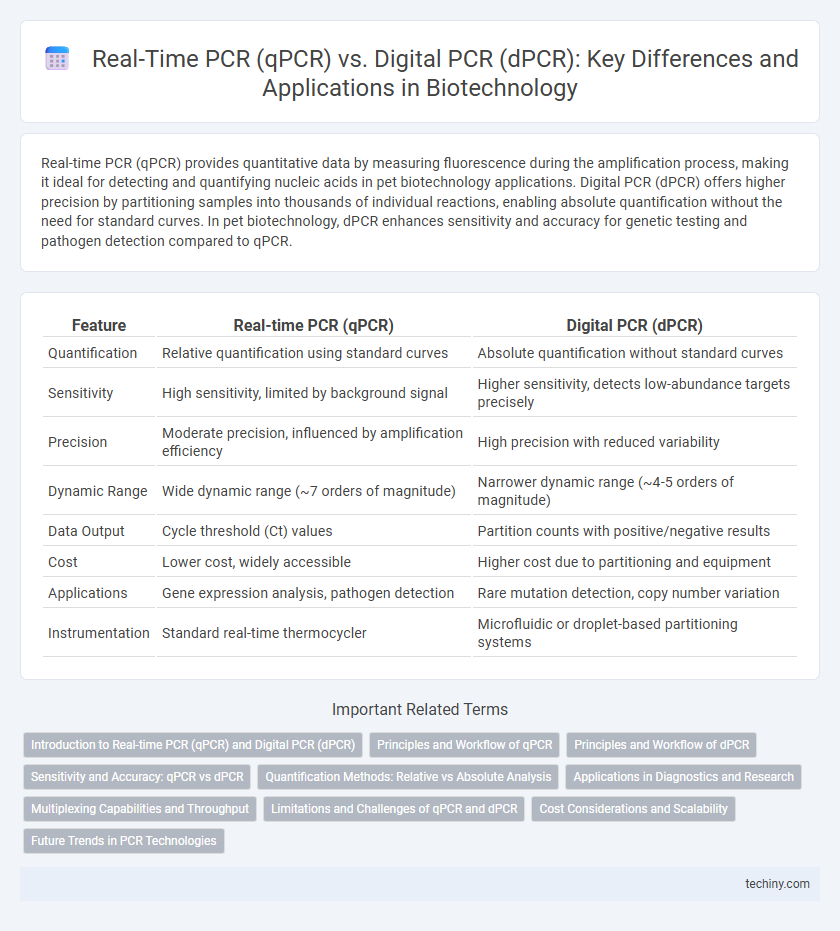
Table of Comparison
| Feature | Real-time PCR (qPCR) | Digital PCR (dPCR) |
|---|---|---|
| Quantification | Relative quantification using standard curves | Absolute quantification without standard curves |
| Sensitivity | High sensitivity, limited by background signal | Higher sensitivity, detects low-abundance targets precisely |
| Precision | Moderate precision, influenced by amplification efficiency | High precision with reduced variability |
| Dynamic Range | Wide dynamic range (~7 orders of magnitude) | Narrower dynamic range (~4-5 orders of magnitude) |
| Data Output | Cycle threshold (Ct) values | Partition counts with positive/negative results |
| Cost | Lower cost, widely accessible | Higher cost due to partitioning and equipment |
| Applications | Gene expression analysis, pathogen detection | Rare mutation detection, copy number variation |
| Instrumentation | Standard real-time thermocycler | Microfluidic or droplet-based partitioning systems |
Introduction to Real-time PCR (qPCR) and Digital PCR (dPCR)
Real-time PCR (qPCR) quantifies nucleic acids by measuring fluorescence during the exponential phase of amplification, providing dynamic data with high sensitivity and specificity. Digital PCR (dPCR) partitions the sample into thousands of individual reactions, enabling absolute quantification of DNA copies without reliance on standard curves. Both techniques are essential in biotechnology for applications such as gene expression analysis, mutation detection, and precise quantification of low-abundance targets.
Principles and Workflow of qPCR
Real-time PCR (qPCR) quantifies DNA by amplifying target sequences and measuring fluorescence emitted during each cycle, using sequence-specific probes or intercalating dyes. The qPCR workflow involves sample preparation, DNA denaturation, annealing of primers, extension by DNA polymerase, and real-time fluorescence detection to monitor amplification kinetics. This method enables quantitative analysis in a single reaction, providing dynamic data on nucleic acid concentration with high sensitivity and specificity.
Principles and Workflow of dPCR
Digital PCR (dPCR) partitions a DNA sample into thousands of individual droplets or wells, enabling absolute quantification by counting positive reactions without the need for standard curves. Each partition undergoes PCR amplification independently, and fluorescence signals indicate the presence or absence of target DNA, allowing precise measurement of low-abundance sequences. The workflow of dPCR includes sample preparation, partitioning, PCR amplification, and endpoint fluorescence analysis, offering high sensitivity and quantification accuracy compared to real-time PCR (qPCR).
Sensitivity and Accuracy: qPCR vs dPCR
Digital PCR (dPCR) offers superior sensitivity and absolute quantification by partitioning samples into thousands of micro-reactions, enabling detection of rare targets and subtle genetic variations often missed by real-time PCR (qPCR). While qPCR relies on amplification cycles and relative quantification, which may introduce variability and limit sensitivity, dPCR provides higher precision and reproducibility without the need for standard curves. This enhanced accuracy makes dPCR invaluable for applications requiring precise quantification, such as detecting low-abundance mutations or viral load measurement.
Quantification Methods: Relative vs Absolute Analysis
Real-time PCR (qPCR) utilizes relative quantification by comparing the amplification of target DNA to a reference gene or standard curve, providing insights into gene expression levels with high throughput. Digital PCR (dPCR) enables absolute quantification by partitioning the sample into thousands of micro-reactions, allowing direct counting of target DNA molecules without the need for standard curves. This fundamental difference enhances dPCR's sensitivity and precision in detecting low-abundance targets and rare mutations in complex biological samples.
Applications in Diagnostics and Research
Real-time PCR (qPCR) enables quantitative analysis of nucleic acids by measuring fluorescence during DNA amplification, making it ideal for viral load monitoring and gene expression studies in diagnostics and research. Digital PCR (dPCR) partitions samples into thousands of micro-reactions, providing absolute quantification without the need for standard curves, which enhances sensitivity and precision in detecting low-abundance mutations and rare genetic variants. Both technologies are pivotal in oncology, infectious diseases, and genetic disorder diagnostics, with dPCR offering superior performance in applications requiring high accuracy and sensitivity.
Multiplexing Capabilities and Throughput
Real-time PCR (qPCR) offers moderate multiplexing capabilities, typically allowing detection of up to 5 targets simultaneously through fluorescent probes, suitable for high-throughput gene expression analysis. Digital PCR (dPCR) enhances multiplexing precision by partitioning the sample into thousands of nanoliter reactions, enabling absolute quantification of multiple targets with higher sensitivity and reduced amplification bias. Throughput in qPCR is generally higher due to faster cycle times and standard plate formats, whereas dPCR throughput is limited by partitioning devices but excels in applications requiring precise quantification of low-abundance targets.
Limitations and Challenges of qPCR and dPCR
Real-time PCR (qPCR) faces limitations such as reduced sensitivity in detecting low-abundance targets and quantification inaccuracies due to amplification efficiency variability. Digital PCR (dPCR) overcomes some qPCR challenges by providing absolute quantification without standard curves, but it encounters limitations like higher cost, longer processing times, and complex data analysis. Both techniques require careful optimization to address issues related to sample quality, inhibitor presence, and dynamic range constraints in precise nucleic acid quantification.
Cost Considerations and Scalability
Real-time PCR (qPCR) offers lower initial instrument costs and faster turnaround times, making it cost-effective for high-throughput applications and routine diagnostics. Digital PCR (dPCR) involves higher upfront expenses due to specialized equipment and reagents, but provides superior precision and sensitivity, which can reduce repeat testing costs in complex or low-abundance target detection. Scalability favors qPCR for large sample volumes owing to established workflows and multiplexing, while dPCR suits niche applications requiring absolute quantification and resistance to PCR inhibitors despite limited throughput.
Future Trends in PCR Technologies
Real-time PCR (qPCR) continues advancing with enhanced multiplexing capabilities and faster thermal cycling, improving quantification accuracy in gene expression and pathogen detection. Digital PCR (dPCR) is evolving towards higher partition densities and integrated microfluidics, enabling ultra-sensitive mutation detection and absolute quantification without standard curves. Future PCR technologies are expected to integrate AI-driven data analysis and miniaturized platforms for point-of-care diagnostics, dramatically increasing throughput and precision in molecular biology applications.
Real-time PCR (qPCR) vs Digital PCR (dPCR) Infographic
 techiny.com
techiny.com