Restriction enzymes act as molecular scissors that cut DNA at specific sequences, enabling precise gene editing in biotechnology pet research. Ligases function as molecular glue, joining DNA fragments together by forming phosphodiester bonds to create recombinant DNA molecules. The combined use of restriction enzymes and ligases facilitates the manipulation of pet genetic material for advancements in genetic engineering and therapeutic development.
Table of Comparison
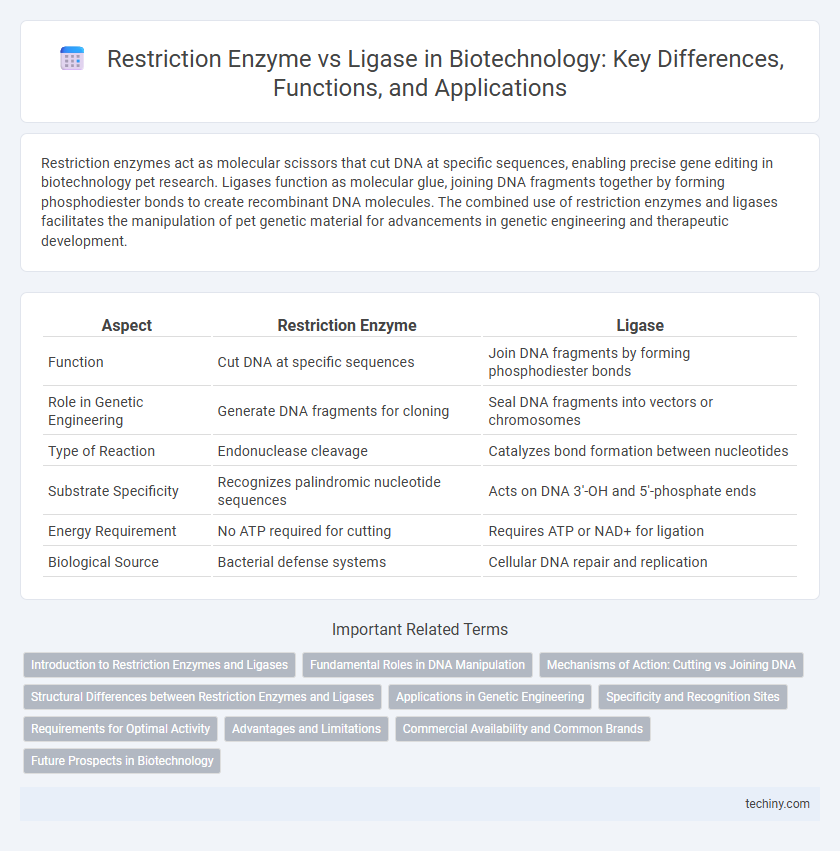
| Aspect | Restriction Enzyme | Ligase |
|---|---|---|
| Function | Cut DNA at specific sequences | Join DNA fragments by forming phosphodiester bonds |
| Role in Genetic Engineering | Generate DNA fragments for cloning | Seal DNA fragments into vectors or chromosomes |
| Type of Reaction | Endonuclease cleavage | Catalyzes bond formation between nucleotides |
| Substrate Specificity | Recognizes palindromic nucleotide sequences | Acts on DNA 3'-OH and 5'-phosphate ends |
| Energy Requirement | No ATP required for cutting | Requires ATP or NAD+ for ligation |
| Biological Source | Bacterial defense systems | Cellular DNA repair and replication |
Introduction to Restriction Enzymes and Ligases
Restriction enzymes, also known as restriction endonucleases, are proteins that cut DNA at specific nucleotide sequences, enabling precise genetic modifications in biotechnology applications. DNA ligases are enzymes that facilitate the joining of DNA strands by catalyzing the formation of phosphodiester bonds, crucial for DNA replication and recombinant DNA technology. Together, restriction enzymes and ligases form the foundation of molecular cloning by enabling the cutting and pasting of DNA fragments.
Fundamental Roles in DNA Manipulation
Restriction enzymes function as molecular scissors that recognize and cleave specific DNA sequences, enabling precise cutting of DNA molecules for genetic analysis and cloning. DNA ligases catalyze the formation of phosphodiester bonds, sealing nicks in the sugar-phosphate backbone and joining DNA fragments together to create continuous strands. Together, these enzymes facilitate essential processes in recombinant DNA technology, such as gene splicing and molecular cloning.
Mechanisms of Action: Cutting vs Joining DNA
Restriction enzymes function by recognizing specific palindromic DNA sequences and cleaving the phosphodiester bonds, generating either sticky or blunt ends for precise DNA fragmentation. Ligases catalyze the formation of phosphodiester bonds between adjacent nucleotides, effectively joining DNA fragments and repairing discontinuities in the sugar-phosphate backbone. The complementary action of restriction enzymes and ligases underpins key molecular cloning techniques, enabling targeted DNA modification and recombinant DNA construction.
Structural Differences between Restriction Enzymes and Ligases
Restriction enzymes are typically homodimeric proteins with symmetrical structures that recognize specific palindromic DNA sequences and cleave phosphodiester bonds, creating sticky or blunt ends. In contrast, DNA ligases are monomeric or heterodimeric enzymes possessing an ATP- or NAD+-binding domain essential for catalyzing the formation of phosphodiester bonds between adjacent nucleotides during DNA repair or replication. Structural differences between these enzymes reflect their distinct functions: restriction enzymes have DNA-binding clefts optimized for sequence-specific cleavage, while ligases have flexible active sites designed for strand joining and sealing nicks in the DNA backbone.
Applications in Genetic Engineering
Restriction enzymes enable precise cutting of DNA at specific sequences, facilitating gene splicing and cloning in genetic engineering. Ligases join DNA fragments by forming phosphodiester bonds, essential for constructing recombinant DNA molecules and repairing DNA strands. Together, these enzymes are crucial tools for gene manipulation, vector construction, and synthetic biology applications.
Specificity and Recognition Sites
Restriction enzymes exhibit high specificity by recognizing precise palindromic DNA sequences, typically 4-8 base pairs long, enabling targeted cleavage of double-stranded DNA at defined recognition sites. Ligases, in contrast, do not recognize specific sequences but function to catalyze the formation of phosphodiester bonds between adjacent nucleotides, facilitating the joining of DNA fragments regardless of sequence context. The specificity of restriction enzymes is crucial for genetic engineering applications, while ligases provide the essential enzymatic activity required for DNA repair and recombinant DNA molecule construction.
Requirements for Optimal Activity
Restriction enzymes require specific DNA sequences, optimal temperature (usually 37degC), and a buffered environment with appropriate ionic strength and pH for optimal activity. DNA ligases demand ATP or NAD+ as cofactors, a slightly different pH range, and divalent cations such as Mg2+ to efficiently catalyze phosphodiester bond formation. Both enzymes depend on precise reaction conditions to maintain structural integrity and catalytic efficiency during molecular cloning processes.
Advantages and Limitations
Restriction enzymes offer precise DNA cutting at specific recognition sites, facilitating targeted gene manipulation with high specificity; however, their limitation lies in sequence dependence, restricting their applicability to known or accessible sites. Ligases provide the advantage of sealing nicks and joining DNA fragments efficiently, enabling the construction of recombinant DNA molecules; their effectiveness can be hindered by suboptimal reaction conditions and the need for compatible DNA ends. Optimizing the combined use of restriction enzymes and ligases enhances molecular cloning efficiency by balancing precision cleavage with reliable fragment assembly.
Commercial Availability and Common Brands
Restriction enzymes such as EcoRI, HindIII, and BamHI are widely available from major suppliers including New England Biolabs, Thermo Fisher Scientific, and Sigma-Aldrich, ensuring consistent quality for DNA cleavage applications. DNA ligases like T4 DNA ligase are commercially accessible through the same leading brands, offering reliable options for DNA fragment joining in cloning and recombinant DNA projects. These companies provide extensive product catalogs and technical support, making them the top choices for researchers in molecular biology and genetic engineering.
Future Prospects in Biotechnology
Restriction enzymes and ligases play crucial roles in genetic engineering, enabling precise DNA manipulation that drives innovations in synthetic biology and gene therapy. Advances in enzyme engineering are expected to enhance specificity and efficiency, facilitating the development of novel biotechnological applications such as personalized medicine and sustainable bio-manufacturing. Emerging technologies like CRISPR, combined with optimized restriction enzymes and ligases, promise to revolutionize genome editing by improving accuracy and reducing off-target effects.
Restriction enzyme vs Ligase Infographic
 techiny.com
techiny.com