Enzyme immobilization in biotechnology pet applications enhances enzyme stability, allowing repeated use and reducing overall costs compared to free enzymes. Immobilized enzymes exhibit improved resistance to environmental changes such as pH and temperature fluctuations, ensuring consistent performance in pet-related biochemical processes. In contrast, free enzymes often suffer from rapid degradation and limited reuse, making immobilization a preferred strategy for sustainable and efficient pet biotechnology solutions.
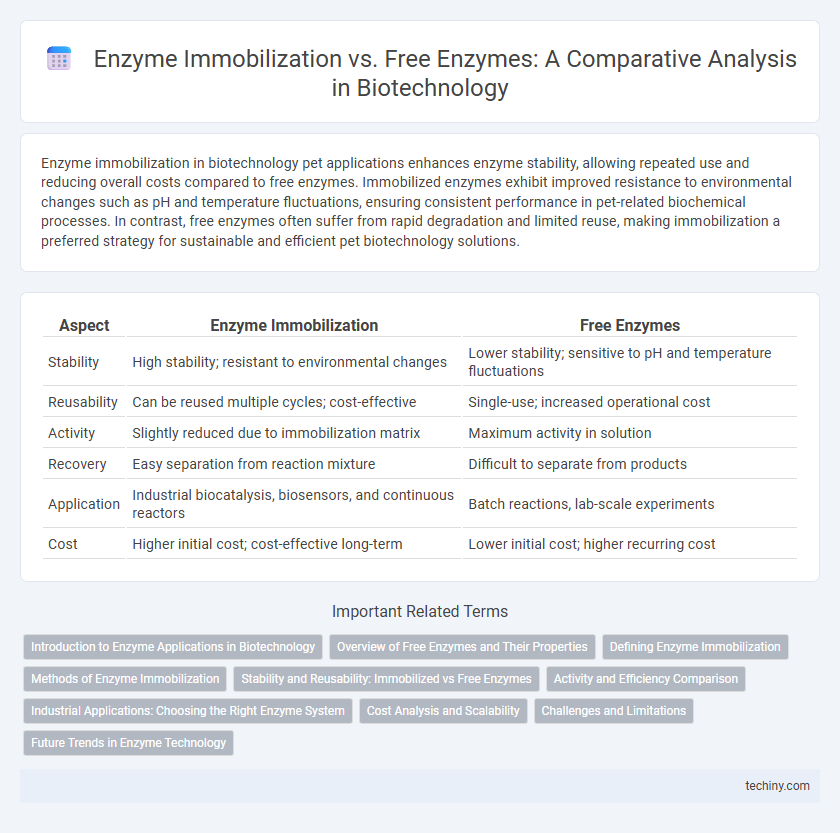
Table of Comparison
| Aspect | Enzyme Immobilization | Free Enzymes |
|---|---|---|
| Stability | High stability; resistant to environmental changes | Lower stability; sensitive to pH and temperature fluctuations |
| Reusability | Can be reused multiple cycles; cost-effective | Single-use; increased operational cost |
| Activity | Slightly reduced due to immobilization matrix | Maximum activity in solution |
| Recovery | Easy separation from reaction mixture | Difficult to separate from products |
| Application | Industrial biocatalysis, biosensors, and continuous reactors | Batch reactions, lab-scale experiments |
| Cost | Higher initial cost; cost-effective long-term | Lower initial cost; higher recurring cost |
Introduction to Enzyme Applications in Biotechnology
Enzyme immobilization enhances enzyme stability, reusability, and operational efficiency compared to free enzymes, making it crucial for industrial biotechnology processes such as biocatalysis, biosensors, and biofuel production. Immobilized enzymes often exhibit increased resistance to environmental changes like pH and temperature, improving process control and reducing costs. Free enzymes, while offering higher catalytic activity, face limitations in recovery and reuse, which hamper scalability in commercial applications.
Overview of Free Enzymes and Their Properties
Free enzymes exhibit high catalytic activity and specificity due to their unbound state, allowing rapid substrate access and efficient turnover rates in biochemical reactions. However, their instability under varying pH, temperature, and operational conditions limits reuse and industrial applicability. Their solubility facilitates homogeneous reaction environments but poses challenges in separation and recovery after catalysis.
Defining Enzyme Immobilization
Enzyme immobilization involves attaching enzymes to solid supports or within matrices to enhance stability, reusability, and ease of separation from reaction mixtures, which significantly improves industrial process efficiency. Unlike free enzymes that freely float and are often less stable and harder to recover, immobilized enzymes maintain catalytic activity under varied conditions and enable continuous operation. This technique is fundamental in biotechnology for optimizing enzyme performance in pharmaceutical synthesis, food processing, and biofuel production.
Methods of Enzyme Immobilization
Enzyme immobilization methods include physical adsorption, covalent bonding, entrapment, and cross-linking, each offering distinct advantages in stability and reusability compared to free enzymes. Physical adsorption relies on weak interactions, while covalent bonding ensures strong attachment to supports like silica or polymers, enhancing enzyme durability in industrial biocatalysis. Entrapment in gels or membranes restricts enzyme mobility but protects activity, and cross-linking forms enzyme aggregates that improve operational stability in processes such as pharmaceutical synthesis and biofuel production.
Stability and Reusability: Immobilized vs Free Enzymes
Enzyme immobilization significantly enhances enzyme stability by restricting conformational changes and protecting enzymes from harsh environmental conditions, leading to prolonged operational lifespan compared to free enzymes. Immobilized enzymes demonstrate superior reusability due to their fixed location on solid supports, enabling repeated use without substantial loss of activity, unlike free enzymes that often degrade or denature after a single cycle. The improved stability and reusability of immobilized enzymes make them highly advantageous for industrial biocatalysis, reducing operational costs and increasing process efficiency.
Activity and Efficiency Comparison
Enzyme immobilization enhances catalytic activity by stabilizing the enzyme structure, allowing repeated use and improving operational stability compared to free enzymes. Immobilized enzymes often exhibit higher efficiency in industrial processes due to increased resistance to pH and temperature variations, reducing enzyme degradation. Free enzymes demonstrate higher initial activity but suffer rapid loss of function, limiting their reusability and overall process efficiency.
Industrial Applications: Choosing the Right Enzyme System
Enzyme immobilization enhances stability, reusability, and control over reaction conditions, making it ideal for continuous industrial processes such as biofuel production and pharmaceutical synthesis. Free enzymes offer higher initial activity and flexibility, suitable for batch processes requiring rapid substrate conversion and diverse reaction environments. Selecting the appropriate enzyme system depends on process scale, cost efficiency, and product specificity within industrial biotechnology applications.
Cost Analysis and Scalability
Enzyme immobilization offers significant cost advantages over free enzymes by enabling enzyme reuse, reducing the frequency of enzyme replacement in industrial processes. Immobilized enzymes enhance scalability due to their improved stability and resistance to harsh operational conditions, facilitating continuous production in large-scale bioreactors. Free enzymes, while initially cheaper, often incur higher long-term costs and limited scalability caused by enzyme denaturation and rapid activity loss.
Challenges and Limitations
Enzyme immobilization enhances enzyme stability and reusability but faces challenges such as mass transfer limitations and potential loss of catalytic activity due to conformational changes. Free enzymes offer higher catalytic efficiency and easier substrate access but suffer from poor stability and cannot be reused effectively in industrial processes. Overcoming these limitations requires optimizing immobilization techniques to balance enzyme activity retention with operational durability.
Future Trends in Enzyme Technology
Enzyme immobilization offers enhanced stability, reusability, and resistance to harsh conditions compared to free enzymes, driving future advancements in biocatalysis and industrial biotechnology. Emerging trends include the use of nanomaterials and microencapsulation techniques to improve enzyme loading efficiency and catalytic performance. Integration of immobilized enzymes in continuous flow reactors and biosensors will further expand their applications in pharmaceuticals, biofuels, and environmental monitoring.
Enzyme Immobilization vs Free Enzymes Infographic
 techiny.com
techiny.com