Fermentation and cell culture are essential biotechnological techniques for producing biologics in pet healthcare. Fermentation utilizes microbial cells like bacteria or yeast to generate large quantities of proteins or enzymes efficiently, while cell culture involves growing animal or insect cells to produce complex proteins with accurate post-translational modifications. Choosing between fermentation and cell culture depends on the product's complexity, scalability needs, and cost considerations in pet biotechnology applications.
Table of Comparison
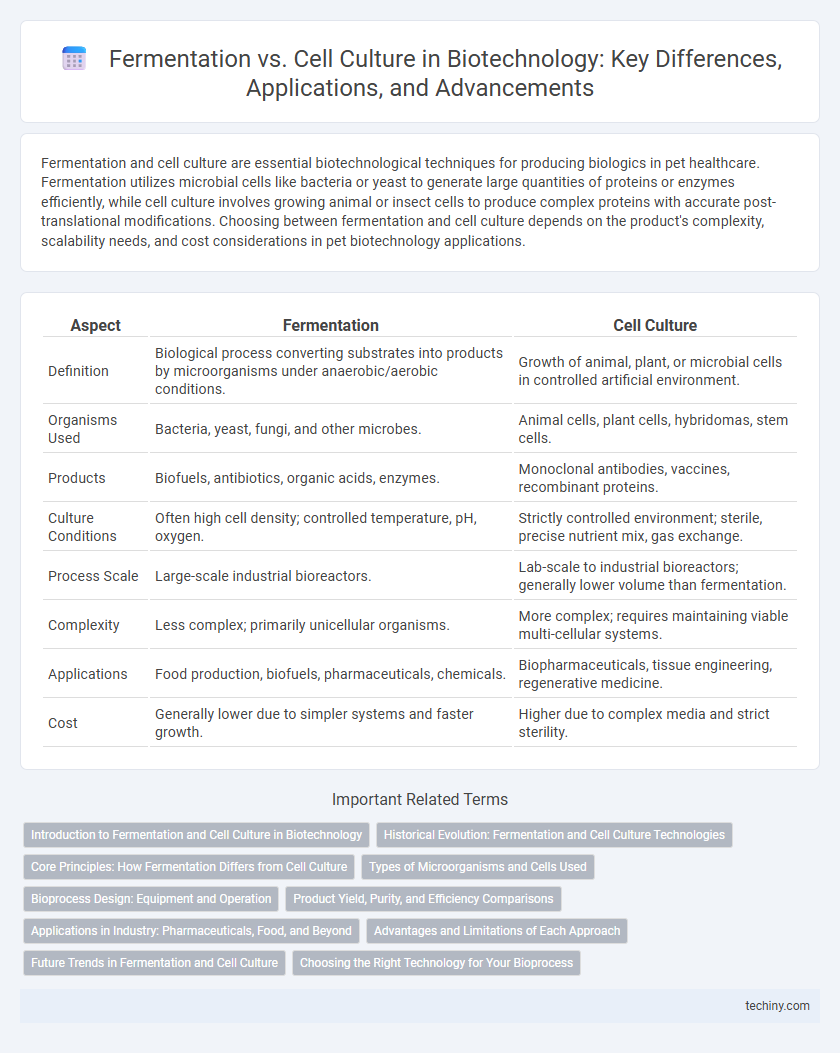
| Aspect | Fermentation | Cell Culture |
|---|---|---|
| Definition | Biological process converting substrates into products by microorganisms under anaerobic/aerobic conditions. | Growth of animal, plant, or microbial cells in controlled artificial environment. |
| Organisms Used | Bacteria, yeast, fungi, and other microbes. | Animal cells, plant cells, hybridomas, stem cells. |
| Products | Biofuels, antibiotics, organic acids, enzymes. | Monoclonal antibodies, vaccines, recombinant proteins. |
| Culture Conditions | Often high cell density; controlled temperature, pH, oxygen. | Strictly controlled environment; sterile, precise nutrient mix, gas exchange. |
| Process Scale | Large-scale industrial bioreactors. | Lab-scale to industrial bioreactors; generally lower volume than fermentation. |
| Complexity | Less complex; primarily unicellular organisms. | More complex; requires maintaining viable multi-cellular systems. |
| Applications | Food production, biofuels, pharmaceuticals, chemicals. | Biopharmaceuticals, tissue engineering, regenerative medicine. |
| Cost | Generally lower due to simpler systems and faster growth. | Higher due to complex media and strict sterility. |
Introduction to Fermentation and Cell Culture in Biotechnology
Fermentation in biotechnology involves the use of microorganisms such as bacteria, yeast, or fungi to convert organic substrates into valuable products like antibiotics, enzymes, and biofuels through anaerobic or aerobic metabolic processes. Cell culture refers to the growth and maintenance of animal, plant, or microbial cells under controlled conditions, enabling the production of biopharmaceuticals, vaccines, and recombinant proteins. Both techniques are fundamental for large-scale production, with fermentation primarily leveraging microbial bioreactors while cell culture typically employs mammalian or insect cell lines in sterile environments.
Historical Evolution: Fermentation and Cell Culture Technologies
Fermentation technology dates back thousands of years, with early civilizations utilizing microbial processes for bread, beer, and cheese production, laying foundational principles for modern biotechnological applications. Cell culture techniques emerged prominently in the 20th century, revolutionizing biomedical research by enabling the growth of mammalian cells in vitro for drug development, vaccine production, and therapeutic research. The historical evolution of these technologies highlights a transition from traditional, large-scale microbial fermentation to sophisticated, controlled cell culture systems pivotal for advanced biomanufacturing.
Core Principles: How Fermentation Differs from Cell Culture
Fermentation relies on microbial metabolism under anaerobic or aerobic conditions to produce bio-based products through substrate conversion, emphasizing large-scale, rapid biomass and metabolite generation. Cell culture involves the growth of animal, plant, or insect cells in controlled environments to facilitate complex protein synthesis and cellular function studies, focusing on maintaining sterile conditions and precise nutrient composition. The key difference lies in fermentation's use of microorganisms for biochemical transformations versus cell culture's reliance on eukaryotic cells for intricate biological processes.
Types of Microorganisms and Cells Used
Fermentation primarily utilizes microorganisms such as bacteria, yeast, and fungi to produce bio-products through anaerobic or aerobic metabolic processes. Cell culture involves the growth of eukaryotic cells, including mammalian, insect, or plant cells, under controlled aseptic conditions for the production of complex proteins and biologics. The choice between fermentation and cell culture depends on the desired product, with fermentation favoring simpler microbial systems and cell culture enabling the production of sophisticated therapeutic compounds.
Bioprocess Design: Equipment and Operation
Fermentation systems utilize bioreactors designed for microbial growth under controlled parameters such as pH, temperature, and aeration, optimizing metabolic activity for product yield. Cell culture bioprocesses require sophisticated equipment like stirred-tank bioreactors with precise control over oxygen transfer rates and shear stress to maintain mammalian or insect cell viability. Both processes demand tailored operational strategies, including sterilization protocols and monitoring systems, to ensure consistent quality and scalability in biopharmaceutical manufacturing.
Product Yield, Purity, and Efficiency Comparisons
Fermentation processes typically yield higher product concentrations due to microbial growth rates and substrate conversion efficiency, making them suitable for large-scale production of biomolecules like antibiotics and enzymes. Cell culture, often used for complex proteins such as monoclonal antibodies, provides superior product purity and post-translational modifications but generally exhibits lower yields and higher operational costs. Efficiency in fermentation is enhanced by rapid biomass accumulation and simpler nutrient requirements, whereas cell culture demands stringent environmental control and longer production times, impacting overall process scalability.
Applications in Industry: Pharmaceuticals, Food, and Beyond
Fermentation processes dominate pharmaceutical production by enabling large-scale synthesis of antibiotics, vaccines, and insulin through microbial metabolism. Cell culture techniques are critical in biopharmaceuticals for producing monoclonal antibodies, recombinant proteins, and gene therapies with high specificity and purity. In the food industry, fermentation enhances flavor, preservation, and nutritional value in products like yogurt and cheese, while cell culture innovation supports alternative protein development and sustainable food technologies.
Advantages and Limitations of Each Approach
Fermentation offers high cell density and scalability, making it ideal for producing large quantities of bioproducts such as antibiotics and enzymes, but it is limited by the need for precise control over anaerobic or aerobic conditions and can generate unwanted byproducts. Cell culture enables the growth of mammalian or insect cells for producing complex proteins and biologics with proper post-translational modifications, yet it often involves higher costs, slower growth rates, and susceptibility to contamination. Both techniques require optimization of environmental parameters such as pH, temperature, and nutrient supply to maximize yield and product quality in biomanufacturing applications.
Future Trends in Fermentation and Cell Culture
Emerging trends in fermentation and cell culture emphasize continuous bioprocessing and integration of real-time monitoring technologies such as sensor-driven automation to enhance productivity and product consistency. Advances in synthetic biology and metabolic engineering are driving the development of tailored microbial and mammalian cell lines, optimizing yield and functionality for pharmaceuticals, biofuels, and food industries. Furthermore, sustainable bioprocessing using renewable feedstocks and waste valorization is increasingly prioritized to reduce environmental impact and improve economic viability.
Choosing the Right Technology for Your Bioprocess
Selecting between fermentation and cell culture hinges on the bioprocess goals, with fermentation excelling in large-scale microbial production of enzymes, antibiotics, and biofuels due to its cost-effectiveness and rapid growth rates of microorganisms like E. coli and yeast. Cell culture is preferred for producing complex biologics such as monoclonal antibodies and recombinant proteins, leveraging mammalian cells like CHO (Chinese Hamster Ovary) that provide proper protein folding and post-translational modifications. Process yield, scalability, product complexity, and regulatory compliance are critical parameters influencing the optimal technology choice for achieving efficient and high-quality bioproduction.
Fermentation vs Cell Culture Infographic
 techiny.com
techiny.com